Abstract
Background & objective
Patients in the intensive care unit have a high prevalence of vitamin D deficiency (VDD). In the present study, clinical outcomes in the ICU were analyzed with vitamin D status.
Materials and methods
In this prospective, multicenter study, sampling was conducted on seven ICUs in three hospitals. Within the first 24 h of ICU admission, patient’s serum vitamin D levels were measured, and their disease severity was monitored using the scores of acute physiologic assessment and chronic health evaluation II (APACHE II), sequential organ failure assessment (SOFA), and the modified Nutrition Risk in Critically ill (mNUTRIC) score.
Results
A total of 236 patients were enrolled in this study, of which 163 (69.1%) had lower vitamin D levels than 20 ng/ml upon ICU admission. The patients with VDD had higher APACHE II scores)P = 0.02), SOFA scores (P < 0.001), and mNUTRIC scores (P = 0.01). Patients with sufficient levels of vitamin D (> 30 ng/ml) had a shorter stay at ICU (P < 0.001). VDD was independently associated with 28-day mortality (OR: 4.83; 95% CI: 1.63–14.27; P = 0.004).
Conclusion
The data showed that VDD was common among the critically ill and was related to a more severe course of illness and a higher mortality rate.
Similar content being viewed by others
Introduction
Vitamin D is a fat-soluble prohormone synthesized in the skin in response to sunlight exposure or is received in small amounts through dietary intake [1]. Vitamin D is involved in the local immune responses to pathogens and the inflammatory pathways of systemic infections. Vitamin D deficiency (VDD) is associated with an increased risk of sepsis in critically ill patients [2, 3]. According to the literature, normal vitamin D levels are defined based on serum cholecalciferol levels higher than 30 ng/ml, while lower serum levels than 30 ng/l represent vitamin D insufficiency; deficiency generally refers to lower levels serum levels than 20 ng/l [4]. Generally, evidence indicates that VDD is a major nutritional challenge for Iranian authorities, as the prevalence of VDD in Iran's population has been reported to be up to 98% [5, 6].
Serum vitamin D levels in healthy individuals depend on several factors, including diet, sunlight exposure, body fat percentage, and melanin content in the skin. Typically, critically ill patients have several problems and comorbidities, such as acute pancreatitis, multiple organ failure, critically ill obesity, surgical procedure, trauma, and sepsis. In these patients, immobility, fluid retention, inflammation, and renal conditions deteriorate their condition and cause deficiency more frequently than in the average population [7, 8]. The function of clinical nutrition in alleviating and managing the morbidities of patients is crucial [9]. In the critical care setting, VDD is associated with adverse outcomes such as infections, increased length of hospital stay, acute kidney injury, and higher mortality [10, 11]. Vitamin D plays a vital role in the immune system through its receptors on various immune cells, including activated CD4 and CD8 T cells, B cells, macrophages, neutrophils, and dendritic cells. Vitamin D also regulates the production of immunoglobulins [12].
VDD in patients with acute conditions is associated with infections, the progression of sepsis and acute respiratory distress syndrome, and increased mortality [3]. The association between vitamin D concentration and ICU outcomes was also previously assessed in some studies [13, 14]. However, the role of vitamin D in critically ill patients remains unclear [15]; thus, a multicenter study with a high number of patients compared to prior samples may provide reliable results for further investigation. In addition, it is still unknown whether VDD in patients admitted to the intensive care unit (ICU) indicates the severity of the disease or is a significant cause of mortality with direct effects [16]. The current study set out to examine the correlations between plasma vitamin D levels, clinical outcomes, and mortality in 236 patients admitted to the ICU.
Materials and methods
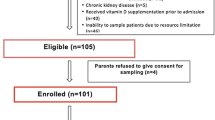
This prospective, observational study was performed in four medical, one surgical, and two trauma ICUs in Mashhad, Iran, during July 2019-March 2020. A convenience sample of the critically ill patients admitted to the ICU was enrolled. The inclusion criterion of the study was the age of more than 18 years, and the exclusion criteria were (i) pregnant women, (ii) receiving 50,000 units of vitamin D 2–4 months before ICU admission, and (iii) receiving multivitamin supplements during/before the ICU admission.
Venous blood samples were collected from the patients within 24 h after ICU admission to measure the plasma levels of 25-hydroxyvitamin D. Plasma samples were collected and preserved at -80°C until the measurements. The second-generation electrochemiluminescence technology platform assessed 25(OH) D, and the results were expressed as ng/ml.
In compliance with the guidelines of the Institute of Medicine, Food, and Nutrition Board [4], the patients were stratified into three groups based on their serum 25(OH)D levels, as follows:
-
1.
Normal group with vitamin D concentrations of > 30 ng/ml (75 nmol/l);
-
2.
Insufficient group with vitamin D concentrations of 20–30 ng/ml (50–75 nmol/l);
-
3.
Deficient group with vitamin D concentrations of < 20 ng/ml (50 nmol/l).
For the assessment of the severity of illness and mortality prediction in the ICUs, acute physiologic assessment and chronic health evaluation (APACHE II) (Additional file 1.docx: Table S1) and sequential organ failure assessment (SOFA) (Additional file 1.docx: Table S2) was applied. APACHE ӀӀ score included variables such as mean blood pressure, heart rate, temperature, respiratory rate and Glasgow Coma Score, white blood cell count, hematocrit, potassium, sodium, creatinine, serum Pa02 and pH, severe failure organs, and age [17].
The SOFA score is based on the degree of dysfunction of six organ systems, including the respiratory, liver, cardiovascular, coagulation, renal, and central nervous systems [18].
The NUTRIC, or the Nutrition Risk in the Critically Ill Score, is a helpful tool for assessing nutritional risk in ICU patients. This Score included six variables including age, number of comorbidities, APACHE II score, SOFA score, serum interleukin 6 (IL-6) concentration, and number of days in the hospital before admission to the ICU. Nevertheless, IL-6, an inflammatory marker, is not commonly measured in the ICUs, so a modified NUTRIC Score (mNUTRIC) without IL-6 (Additional file 1.docx: Table S3) was used. Patients with a score ≥ 5 were considered at high nutritional risk [19].
The collected data was included demographic characteristics (age, gender), comorbidities, cause of admission, ideal body weight, mechanical ventilation, and length of ICU stay.
APACHE II and mNUTRIC questionnaires were filled out within 24 h after ICU admission.
SOFA score was calculated every two days until ICU discharge or for a maximum of 7 days.
Anthropometric assessment, including weight (There are several methods to calculate the ideal weight. One of the best and easiest methods is to use height and the difference of 100 for men and 102 for women) [20], height (via the ulna length), and body mass index, were measured at baseline.
In addition, these patients' mid-arm circumference (MAC) was measured using an inelastic meter with an accuracy of 0.5 cm.
The mortality rate was asked by phone within 28 days of the start of the study.
Sample size
The sample size was calculated in accordance with a previous study [13], and the length of ICU stay was used as the main variable. Based on the formula of comparing quantitative variables in two groups for the length of ICU stay and the Type I error of 5% (α = 0.05) and Type II error of 20% (β = 0.20; the power of the study was 80%) and with a 15% probability of drop-out patients during the study, the total sample size was estimated about 236 patients.
Ethical considerations
The Ethics Committee of Mashhad University of Medical Sciences approved this work with the code: IR.MUMS.MEDICAL.REC.1398.086. Written informed consent was obtained from all patients before participating in the study. However, since some ICU patients were severely ill or unconscious, their close relatives were asked to fill out the informed consent forms before beginning the study.
Statistical analysis
Data analysis was performed in SPSS version 11.5 (SPSS Inc., IBM Company, Chicago, IL). The normality of the distribution of variables was evaluated using the Kolmogorov–Smirnov test, and normally distributed variables were expressed as mean and standard deviation [21]. The qualitative data were reported as frequency and percentages. Variables with abnormal distribution were expressed as median (25th quartile, 75th quartile). The correlation of each variable with the outcome categories (normal, insufficiency, and vitamin D deficiency) was evaluated separately using χ2 for the categorical variables and the analysis of variance (ANOVA) with post-hoc and Kruskal–Wallis tests for the continuous variables. To determine the combination of the predictive variables for the optimal predictive model, univariate and multivariate binary logistic regression analyses were carried out. The multivariate analysis results were presented as a mean difference with 95% confidence intervals (CI). A value of P less than 0.05 was used as statistically significant.
Results
Patients and outcomes
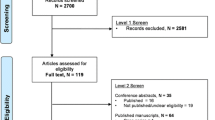
In this study, 250 critically ill patients were enrolled, but the statistical analysis was conducted with 236 participants (150 males and 86 females) (Fig. 1). The baseline characteristics of patients are presented in Table 1.
The mean age of the patients was 44.74 ± 16 years, and the age comparison between deficient (45.73 ± 17.11), insufficient (38.08 ± 15.99), and normal vitamin D (46.41 ± 16.95) levels showed a significant difference (P = 0.04).
The APACHEӀӀ, SOFA, and mNUTRIC scores at baseline were compared between the three groups of VDD, vitamin D insufficiency, and normal vitamin D. According to the obtained results, the severity of illness scores, including APACHE II score (P = 0.02) and SOFA score (P < 0.001) were significantly higher in patients with VDD (15.41 ± 6.49 and 6.77 ± 2.86, respectively) compared with insufficient (13.02 ± 4.85 and 4.93 ± 2.67, respectively), and normal vitamin D (13.02 ± 6.26 and 4.50 ± 3.31, respectively). Also, the mNUTRIC score comparison between the three groups (VDD: 2.76 ± 1.96, insufficient: 1.70 ± 1.50, normal: 1.76 ± 1.95) showed that the patients with VDD had higher nutritional risk (P = 0.01).
In addition, the mean duration of mechanical ventilation time (day) (P = 0.01), prolonged ICU stay (P < 0.001), and eGFR (P < 0.05) were significantly higher in the patients with VDD compared to vitamin D insufficiency and the normal group (Table 1).
All-cause 28-day mortality was estimated at 35.6% in patients with VDD, while 17.6% and 10.3% were in the vitamin D insufficiency and normal groups, respectively, and this relation was significant (P = 0.002) (Fig. 2).
Using binary multivariate logistic regression, the significant outcome predictor was determined. It contained severity rating and vitamin D concentration. According to the findings, VDD increased the risk of 28-day mortality by 4.83 folds compared to normal vitamin D levels. Analyses of logistic regression revealed that VDD was an independent predictor of 28-day mortality. (OR: 4.83; 95% CI: 1.63–14.27) (Table 2).
Discussion
This present study was conducted to assess the correlation between vitamin D concentration and clinical outcomes in 236 ICU patients. Our investigation revealed that 69.1% ICU-admitted patients had low 25-hydroxy D levels at baseline. In addition, it is shown that VDD had a considerable correlation with APACHE II, SOFA, and mNUTRIC scores. Finally, vitamin D level on admission had a notable association with the 28-day mortality rate. All of these findings indicated that early evaluation of vitamin D in critically ill patients and also supplementation with vitamin D might have beneficial effects on these patients and may reduce the rate of 28-day mortality among ICU patients.
The results of this study exhibited that 69.1% of the patients admitted to the ICU had 25-hydroxy VDD, which is a higher rate in comparison to the reports of the previous studies in other countries (20–40%) [22,23,24]. Meanwhile, 80% of participants in Arnson' et al. study [25] and 80.4% of patients in Azim's survey [26], 93.5% in Vosoughi et al.'s study [13] had low baseline serum 25(OH) vitamin D.
In our study, the mean SOFA and APACHEӀӀ scores (predictors of mortality and disease severity) and the mNUTRIC score were higher in the vitamin D deficient group than the group with normal serum vitamin D levels. This is consistent with the study by Anwar et al., which indicated the higher APACHE II score among the vitamin D-deficient group [27]. Another study also showed a higher SOFA score in the group with serum 25(OH)D levels of ≤ 10 ng/ml compared to 25(OH)D levels of > 10 ng/mL [28]. Furthermore, Aygencel et al. reported that the APACHE II and SOFA scores were higher in critically ill patients with vitamin D insufficiency [29]. A cohort study was conducted by Cecchi et al. to evaluate the correlation between vitamin D levels in septic patients and the clinical outcomes, and a clear correlation was reported between these variables [30].
Moreover, a prospective study conducted by Vosoughi et al. (2016) in Iran investigated the correlations between VDD, clinical outcomes, and mortality in 185 patients admitted to the ICU. The results demonstrated insufficient 25(OH)D levels in the patients. However, no significant correlation was observed between the 25(OH)D levels and clinical outcomes in the mentioned study [13].
According to Jeng et al., critical illness was associated with lower vitamin D levels in critically ill patients compared to healthy controls [31]. Similar to previous studies [21, 22, 32], our findings are in line with the result of Lee et al.[33], which indicated that VDD might increase mortality in patients admitted to the ICU. In addition, Moraes et al. suggested that low vitamin D levels upon ICU admission are an independent risk factor for mortality in critically ill patients[34]. In two separate systematic reviews and meta-analyses, Zhang et al.[3] (OR: 1.76; 95% CI: 1.38–2.24) and Hann et al.[35] (OR: 1.7; 95% CI: 1.49–2.16) reported that VDD is associated with the increased incidence of hospital mortality in critically ill adult patients. Meanwhile, Ralph et al. and Cecchi et al. reported no correlation between low vitamin D levels and increased mortality risk in critically ill patients [30, 36]. The meta-analysis conducted by Zhang et al. showed an association between VDD and mortality in patients admitted to the ICU[3]. In contrast, Haan et al. identified VDD as a risk factor for severe infections and mortality in critically ill patients [35].
A recent study of severe sepsis and septic shock patients indicated no correlation between VDD and 90-day mortality [29]. Although VDD may increase mortality in critically ill patients, the causes remain unknown and could be explained by several mechanisms. The higher mortality rate in critically ill patients with VDD may be due to the dysfunction of endothelial and immune cells and changes in glucose and calcium metabolism [37,38,39]. Endothelial cell dysfunction has been suggested as a potential cause of multiple organ dysfunction syndromes [40, 41]. In addition, VDD may intensify metabolic disorders and impair immune regulation, as reported in critically ill patients, leading to deteriorated clinical outcomes compared to patients with normal vitamin D levels. VDD may also increase the risk of inflammation by suppressing immune reactivity [42, 43]. Vitamin D improves immune responses against infections through the up-regulation of toll-like receptors (TLRs) [42].
Additionally, vitamin D has been reported to decrease the expression of pro-inflammatory cytokines [44] and increase the expression of anti-inflammatory cytokines [45]. Moreover, tissues in critically ill patients require more vitamin D, and VDD may lead to widespread tissue dysfunction in these cases. These mechanisms may clarify increased mortality and explain that it occurs due to systemic inflammatory response, organ failure, and metabolic dysfunction in critically ill patients.
In this study, patients with sufficient vitamin D levels experienced a shorter stay at the ICU. This finding is consistent with the McNally et al. study, where patients with low levels of Vitamin D stayed longer at the ICU and were later discharged compared to the sufficient group [46]. In addition, Zhang et al. and de Haan et al. reported the same results[3, 35]. On the contrary, Venkatram et al. and Aygencel et al. stated a significant relationship between low vitamin D levels and longer ICU stays. Therefore, it is suggested that the current findings could be due to the pleiotropic actions of vitamin D [29, 47].
In the present study, a negative association was observed between the length of mechanical ventilation and VDD in the patients admitted to the ICU. Consistent with our findings, a study conducted in India indicated that VDD increased the length of mechanical ventilation in critically ill patients [48]. Additionally, Quraishi et al. stated that plasma levels of 25(OH)D upon admission to the ICU were inversely associated with the duration of respiratory support [49]. In contrast to our findings, the observational, prospective study by Yaghoobi demonstrated no significant correlation between the length of mechanical ventilation and normal vitamin status in patients with ventilator-associated pneumonia and VDD [50]. Patients who require mechanical ventilation in the ICU often experience cellular changes and respiratory muscle weakness [51,52,53,54] that are exacerbated by factors such as malnutrition, electrolyte abnormalities, and severe infections [55]. Vitamin D is essential to human health, especially for bone and muscle function [56], while limited studies have investigated these effects on respiratory muscles.
This study had the main strength of a multicenter study with a large number of patients, which can improve the reliability of the results. However, several potential limitations should be addressed. First, this cross-sectional study cannot establish a cause-and-effect relationship. Second, some confounding factors, such as the type of disease that could bias the results, were not considered. In addition, the patient's serum level of vitamin D was measured only in the first 24 h of hospitalization, and the changes in the level of vitamin D during the hospitalization of the patients in the intensive care unit were not investigated. Prehospital health conditions such as the BMI influencing the results were not considered. Furthermore, more studies are needed to determine if there is a direct relationship between Vitamin D status and patient outcomes in ICU. For this, larger studies are needed to access reliable results.
Conclusion
According to the results, the prevalence of VDD was high in critically ill patients admitted to the ICU. Moreover, inverse correlations were observed between vitamin D levels, disease severity (assessed by APACHE II and SOFA), and length of ICU stay. Importantly, VDD is the independent predictor of mortality in critically ill patients. Therefore, vitamin D administration may improve clinical outcomes in patients with VDD. However, more interventional studies are suggested to investigate the effect of vitamin D on clinical outcomes in patients admitted to the ICU.
Availability of data and materials
The datasets generated and analyzed during the current study and used for the preparation of the manuscript are included in the article submitted for publication.
References
Bouillon R, Carmeliet G. Vitamin D insufficiency: definition, diagnosis and management. Best Pract Res Clin Endocrinol Metab. 2018;32(5):669–84.
Iraj B, Ebneshahidi A, Askari G. Vitamin D deficiency, prevention and treatment. Int J Prev Med. 2012;3(10):733.
Zhang YP, Wan YD, Sun TW, Kan QC, Wang LX. Association between vitamin D deficiency and mortality in critically ill adult patients: a meta-analysis of cohort studies. Crit Care (London England). 2014;18(6):684.
Del Valle HB, Yaktine AL, Taylor CL, Ross AC. Dietary reference intakes for calcium and vitamin D. 2011.
Tabrizi R, Moosazadeh M, Akbari M, Dabbaghmanesh MH, Mohamadkhani M, Asemi Z, et al. High prevalence of vitamin D deficiency among Iranian population: a systematic review and meta-analysis. Iran J Med Sci. 2018;43(2):125.
Bagherniya M, Khorasanchi Z, Bidokhti MS, Ferns GA, Rezaei M, Ghayour-Mobarhan M, et al. The prevalence of vitamin D deficiency in Iran: a literature review. Curr Nutr Food Sci. 2020;16(7):1015–27.
Yahyapoor F, Keshani M, Sedaghat A, Feizi A, Clark CC, Bagherniya M, et al. The effects of adjunctive treatment with L-carnitine on monitoring laboratory variables in ICU patients: a double-blinded randomized controlled clinical trial. Trials. 2023;24(1):1–8.
Lee P, Eisman JA, Center JR. Vitamin D deficiency in critically ill patients. N Engl J Med. 2009;360(18):1912–4.
Badpeyma M, Malekahmadi M, Sedaghat A, Norouzian Ostad A, Khadem-rezaiyan M, Pahlavani N, et al. Evaluation of energy and protein intakes and clinical outcomes in critically ill patients: cross-sectional study. J Nutr Fasting Health. 2023;11:172.
Amrein K, Lasky-Su JA, Dobnig H, Christopher KB. Metabolomic basis for response to high dose vitamin D in critical Illness. Clin Nutr. 2021;40(4):2053–60.
Sistanian F, Sedaghat A, Badpeyma M, Rezaiyan MK, Moghaddam AB, Ranjbar G, et al. Low plasma vitamin D is associated with higher 28 days mortality and worse clinical outcomes in critically Ill patients: results of a prospective study. Clin Nutr ESPEN. 2023;54:626.
Al-Tarrah K, Hewison M, Moiemen N, Lord JM. Vitamin D status and its influence on outcomes following major burn injury and critical Illness. Burns Trauma. 2018;6:6.
Vosoughi N, Kashefi P, Abbasi B, Feizi A, Askari G, Azadbakht L. The relationship between vitamin D, clinical outcomes and mortality rate in ICU patients: a prospective observational study. J Res Med Sci. 2016;21:75.
Alizadeh N, Khalili H, Mohammadi M, Abdollahi A. Serum vitamin D levels at admission predict the length of intensive care unit stay but not in-hospital mortality of critically ill surgical patients. J Res Pharm Pract. 2015;4(4):193.
Langlois PL, Szwec C, D’Aragon F, Heyland DK, Manzanares W. Vitamin D supplementation in the critically ill: a systematic review and meta-analysis. Clin Nutr. 2018;37(4):1238–46.
Insuasty CCB, Guzmán LFC, Ibarra BFS, Ordóñez RRC, Ojeda JCC, Cassiani MAD, et al. Vitamin D deficiency as a risk factor for mortality in critically Ill patients. Health Sci J. 2022;16(3):1–4.
Knaus WA, Zimmerman JE, Wagner DP, Draper EA, Lawrence DE. APACHE—acute physiology and chronic health evaluation: a physiologically based classification system. Crit Care Med. 1981;9(8):591–7.
Vincent J-L, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, et al. The SOFA (Sepsis-related Organ failure Assessment) score to describe organ dysfunction/failure: on behalf of the Working Group on Sepsis-related problems of the European Society of Intensive Care Medicine (see contributors to the project in the appendix). Springer-Verlag; 1996.
Rahman A, Hasan RM, Agarwala R, Martin C, Day AG, Heyland DK. Identifying critically-ill patients who will benefit most from nutritional therapy: further validation of the modified NUTRIC nutritional risk assessment tool. Clin Nutr. 2016;35(1):158–62.
Shayesteh F, Poudineh S, Pouryazdanpanah-Kermani M, Sadat Ayoudi S, Norouzy A. Assessment of nutritional intake in intensive care unit patients of Ghaem hospital. Med J Mashhad Univ Med Sci. 2015;58(4):217–24.
Amrein K, Amrein S, Holl A, Waltensdorfer A, Pieber T, Dobnig H, Vitamin D. Parathyroid hormone and serum calcium levels and their association with hospital mortality in critically ill patients. Crit Care. 2010;14:1.
McKinney JD, Bailey BA, Garrett LH, Peiris P, Manning T, Peiris AN. Relationship between vitamin D status and ICU outcomes in veterans. J Am Med Dir Assoc. 2011;12(3):208–11.
Lucidarme O, Messai E, Mazzoni T, Arcade M, Du Cheyron D. Incidence and risk factors of vitamin D deficiency in critically ill patients: results from a prospective observational study. Intensive Care Med. 2010;36(9):1609–11.
Braun A, Chang D, Mahadevappa K, Gibbons FK, Liu Y, Giovannucci E, et al. Association of low serum 25-hydroxyvitamin D levels and mortality in the critically ill. Crit Care Med. 2011;39(4):671.
Arnson Y, Gringauz I, Itzhaky D, Amital H. Vitamin D deficiency is associated with poor outcomes and increased mortality in severely ill patients. QJM. 2012;105(7):633–9.
Azim A, Ahmed A, Yadav S, Baronia AK, Gurjar M, Godbole MM, et al. Prevalence of vitamin D deficiency in critically ill patients and its influence on outcome: experience from a tertiary care centre in North India (an observational study). J Intensive Care. 2013;1(1):1–5.
Anwar E, Hamdy G, Taher E, Fawzy E, Abdulattif S, Attia MH. Burden and outcome of vitamin D deficiency among critically ill patients: a prospective study. Nutr Clin Pract. 2017;32(3):378–84.
Haliloglu M, Bilgili B, Haliloglu O, Yavuz DG, Cinel I. Vitamin D level is associated with mortality predictors in ventilator-associated pneumonia caused by acinetobacter baumannii. J Infect Dev Ctries. 2016;10(06):567–74.
Aygencel G, Turkoglu M, Tuncel AF, Candır BA, Bildacı YD, Pasaoglu H. Is vitamin D insufficiency associated with mortality of critically ill patients? Crit Care Res Pract. 2013;2013:856747.
Cecchi A, Bonizzoli M, Douar S, Mangini M, Paladini S, Gazzini B, et al. Vitamin D deficiency in septic patients at ICU admission is not a mortality predictor. Minerva Anestesiol. 2011;77(12):1184–9.
Jeng L, Yamshchikov AV, Judd SE, Blumberg HM, Martin GS, Ziegler TR, et al. Alterations in vitamin D status and anti-microbial peptide levels in patients in the intensive care unit with sepsis. J Transl Med. 2009;7:1–9.
Weenink J, Oudemans-Van Straaten H, Yap H, Slaats E, Van Der Voort P. High prevalence of severe vitamin D deficiency in intensive care patients. Crit Care. 2010;14(Suppl 1):P588.
Lee P, Nair P, Eisman JA, Center JR. Vitamin D deficiency in the intensive care unit: an invisible accomplice to morbidity and mortality? Intensive Care Med. 2009;35:2028–32.
Moraes RB, Friedman G, Wawrzeniak IC, Marques LS, Nagel FM, Lisboa TC, et al. Vitamin D deficiency is independently associated with mortality among critically ill patients. Clinics. 2015;70:326–32.
de Haan K, Groeneveld A, de Geus HR, Egal M, Struijs A. Vitamin D deficiency as a risk factor for Infection, sepsis and mortality in the critically ill: systematic review and meta-analysis. Crit Care. 2014;18(6):1–8.
Ralph R, Peter JV, Chrispal A, Zachariah A, Dian J, Sebastian T, et al. Supraphysiological 25-hydroxy vitamin D 3 level at admission is associated with Illness severity and mortality in critically ill patients. J Bone Miner Metab. 2015;33:239–43.
Sanaie S, Mahmoodpoor A, Hamishehkar H, Shadvar K, Salimi N, Montazer M, et al. Association between Disease severity and calcium concentration in critically ill patients admitted to intensive care unit. Anesth Pain Med. 2018;8(1):e57583.
Kelly A, Levine MA. Hypocalcemia in the critically ill patient. J Intensive Care Med. 2013;28(3):166–77.
Adikari M, Perera C, Fernando M, Loeb M, Premawansa S, de Silva D. Prevalence of hypocalcemia and its potential value as a biochemical marker in patients with severe dengue infection. J Trop Dis. 2015;4(188):2.
Ostrowski SR, Haase N, Müller RB, Møller MH, Pott FC, Perner A, et al. Association between biomarkers of endothelial injury and hypocoagulability in patients with severe sepsis: a prospective study. Crit Care. 2015;19(1):1–10.
Johansen ME, Johansson PI, Ostrowski SR, Bestle MH, Hein L, Jensen AL, et al. editors. Profound endothelial damage predicts impending organ failure and death in sepsis. Seminars in Thrombosis and hemostasis. Thieme Medical Publishers; 2015.
Arababadi MK, Nosratabadi R, Asadikaram G. Vitamin D and toll like receptors. Life Sci. 2018;203:105–11.
Medrano M, Carrillo-Cruz E, Montero I, Perez-Simon JA. Vitamin D: effect on haematopoiesis and immune system and clinical applications. Int J Mol Sci. 2018;19(9):2663.
Baeke F, Takiishi T, Korf H, Gysemans C, Mathieu C. Vitamin D: modulator of the immune system. Curr Opin Pharmacol. 2010;10(4):482–96.
Hewison M. Vitamin D and the immune system: new perspectives on an old theme. Rheum Dis Clin. 2012;38(1):125–39.
McNally JD, Nama N, O’Hearn K, Sampson M, Amrein K, Iliriani K, et al. Vitamin D deficiency in critically ill children: a systematic review and meta-analysis. Crit Care. 2017;21(1):1–13.
Venkatram S, Chilimuri S, Adrish M, Salako A, Patel M, Diaz-Fuentes G. Vitamin D deficiency is associated with mortality in the medical intensive care unit. Crit Care. 2011;15(6):1–9.
Padhi R, Panda B, Jagati S, Patra SC. Vitamin D status in adult critically ill patients in Eastern India: an observational retrospective study. Lung India. 2014;31(3):212.
Quraishi SA, McCarthy C, Blum L, Cobb JP, Camargo CA Jr. Plasma 25-hydroxyvitamin D levels at initiation of care and duration of mechanical ventilation in critically ill surgical patients. J Parenter Enter Nutr. 2016;40(2):273–8.
Yaghoobi MH, Taher A, Seifrabie MA, Sabahi M, Rahimi-Bashar F. Serum vitamin D level was not associated with severity of ventilator associated Pneumonia. Romanian J Intern Med. 2019;57(1):55–60.
Jaber S, Petrof BJ, Jung B, Chanques G, Berthet J-P, Rabuel C, et al. Rapidly progressive diaphragmatic weakness and injury during mechanical ventilation in humans. Am J Respir Crit Care Med. 2011;183(3):364–71.
Supinski GS, Ann Callahan L. Diaphragm weakness in mechanically ventilated critically ill patients. Crit Care. 2013;17(3):1–17.
Schepens T, Dres M, Heunks L, Goligher EC. Diaphragm-protective mechanical ventilation. Curr Opin Crit Care. 2019;25(1):77–85.
Dres M, Demoule A. Diaphragm dysfunction during weaning from mechanical ventilation: an underestimated phenomenon with clinical implications. Crit Care. 2018;22(1):1–8.
Martin AD, Smith BK, Gabrielli A. Mechanical ventilation, diaphragm weakness and weaning: a rehabilitation perspective. Respir Physiol Neurobiol. 2013;189(2):377–83.
Dzik KP, Kaczor JJ. Mechanisms of vitamin D on skeletal muscle function: oxidative stress, energy metabolism and anabolic state. Eur J Appl Physiol. 2019;119:825–39.
Acknowledgements
We would like to thank all patients, research assistants, and clinicians for their involvement in the study.
Funding
This study is approved and supported by Mashhad University of Medical Sciences with grant number 970978. The institution's role is only in funding this study and not in designing, analyzing, and interpreting outcomes, collecting data, and writing the final manuscript.
Author information
Authors and Affiliations
Contributions
A. Norouzy, F. Sistanian, A. Sedaghat, M. Badpeyma, M. khadem Rezaiyan, and G. Ranjbar equally contributed to the conception and design of the research; A. Norouzy, F. Sistanian, A. Sedaghat, M. khadem Rezaiyan, A. Bagheri Moghaddam and M. Arabi contributed to the acquisition of the data. F. Sistanian and M. Badpeyma drafted the manuscript; F. Sistanian, M. khadem Rezaiyan, G. Ranjbar, and M. Bagherniya contributed to the acquisition and analysis of the data; A. Norouzy, F. Sistanian, A. Sedaghat, M. Badpeyma, M. khadem Rezaiyan, A. Bagheri Moghaddam and M Arabi contributed to the interpretation of the data. M. Badpeyma, G. Ranjbar review, and M. Bagherniya Editing the manuscript. All authors agree to be fully accountable for ensuring the integrity and accuracy of the work and read and approve the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethics committee of Mashhad University of Medical Sciences approved this study with the ethical code IR.MUMS.MEDICAL.REC.1398.086. Written informed consent was received from all subjects. If they lose consciousness and lack the capacity to consent, close relatives fill out the informed consent form before beginning the study. All methods were carried out in accordance with relevant guidelines and regulations or the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
APCHE II (Acute Physiologic and Chronic Health Evaluation II) score content. Table S2. SOFA (Sequential Organ Failure Assessment) score content. Table S3. mNUTRIC (modified Nutrition Risk in Critically ill) score content.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sistanian, F., Sedaghat, A., Badpeyma, M. et al. Low plasma vitamin D is associated with increased 28-day mortality and worse clinical outcomes in critically ill patients. BMC Nutr 10, 6 (2024). https://doi.org/10.1186/s40795-023-00801-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40795-023-00801-1