Abstract
Background
Non-alcoholic fatty liver disease (NAFLD) represents a spectrum of disease ranging from simple fatty liver to non-alcoholic steatohepatitis, cirrhosis, liver cancer and liver failure. NAFLD affects up to 30–40% of adults in Western countries and is directly linked to overweight and obesity. There are no approved drugs to specifically target NAFLD, therefore weight loss achieved through changes in dietary and physical activity behaviours is the recommended management approach. However, achieving and sustaining weight loss is challenging for patients with NAFLD. We developed a NAFLD-specific digital lifestyle intervention (VITALISE) to target changes in dietary and physical activity behaviours of patients with NAFLD to initiate weight loss and weight loss maintenance. This study aims to evaluate the feasibility and acceptability of VITALISE in a secondary care clinical setting.
Methods
A single-centre, one-arm, prospective design will be used to assess the feasibility and acceptability of recruitment, uptake, engagement and completion of VITALISE. Health-related outcomes will be assessed at baseline and 6-months. An interim measure of self-reported weight, physical activity and self-efficacy will be recorded at 12-weeks. Qualitative semi-structured interviews conducted at 6 months follow up will further explore acceptability and feasibility and fidelity of receipt and enactment. The study aims to recruit 35 patients with newly diagnosed NAFLD over a 6-month time period. Eligible patients will have continuous access to VITALISE and monthly tele-coaching support for 6 months prior to follow-up with a hepatologist.
Discussion
VITALISE offers access to evidence and theory-informed tailored dietary and physical activity support for patients with NAFLD. The intervention is designed for use by patients in their own time, outside of the hospital setting to overcome well documented challenges including attending additional appointments, and lack of time during routine appointments to adequately address lifestyle behaviour change. This feasibility study will determine the feasibility of VITALISE to support clinical care delivery.
Trial registration
ISRCTN12893503.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Non-alcoholic fatty liver disease (NAFLD) affects up to 30–40% of adults in Western countries and is the most common liver condition worldwide [1, 2]. It represents a spectrum of liver disease ranging from simple fatty liver through to non-alcoholic steatohepatitis (NASH – liver inflammation), life threatening cirrhosis, liver cancer and liver failure, and has become a common cause of liver transplant. Approximately 40% of patients with NAFLD will develop progressive liver fibrosis and ultimately, 5–11% will develop end-stage liver disease [3, 4].
NAFLD is directly linked to being overweight or obese, usually caused by chronic excess calorie consumption and a lack of physical activity and exercise [5]. Currently, there are no approved drugs to specifically target or treat NAFLD. Weight loss, achieved through change in dietary and physical activity behaviours is the recommended treatment for NAFLD, which can reduce liver fat, inflammation and fibrosis [6, 7]. Evidence-based clinical guidelines for the management of NAFLD state the importance of lifestyle behaviour change in patients with NAFLD, regardless of disease severity [8,9,10]. However, people with NAFLD find achieving sustained weight loss a significant challenge, and clinicians struggle to support patients with NAFLD due to a lack of specific training, interventions, and referral pathways [11,12,13].
We systematically developed an evidence and theory-informed NAFLD-specific digital lifestyle intervention using Intervention Mapping [14]—VITALISE (interVention to promote lIfesTyle change in non-Alcoholic fatty LIver diseaSE) [15]. Intervention Mapping provides a systematic approach to developing theory- and evidence-informed interventions that integrates the requirements of the target population [14]. It provides a step-by-step account of the translation of theory- and evidence-based behaviour change techniques into intervention components, which explicitly target theoretical determinants of behaviour and behaviour change [14]. This process enables replication in terms of development and delivery of interventions and facilitates intervention optimisation and robust evaluation. VITALISE was co-designed with patients and healthcare professionals (HCPs) and addresses the pressing need for a behaviour change programme for people with NAFLD, targeting dietary and physical activity behaviours to initiate weight loss and weight loss maintenance. It provides guideline-recommended structured education and self-regulation tools to facilitate supported self-management. Together these enable patients to plan and track changes, and develop strategies to overcome any barriers encountered, supported by lifestyle coaches.
Aims and objectives
The aim of the study is to evaluate the feasibility and acceptability of VITALISE in the clinical setting within secondary care. This feasibility study will not include formal hypothesis testing because it is not powered to detect clinically meaningful changes in outcomes. The main aim is to gain insight into whether patients find VITALISE acceptable and whether it is feasible to deliver within routine clinical care.
In accordance with NIHR and CONSORT guidance for feasibility studies [16, 17], the primary objectives are to determine recruitment, retention and attrition rates, intervention uptake, engagement, adherence, and follow-up rates and to test the robustness of our data collection procedures. We will collect data on usability and individual patient views on VITALISE via semi-structured interviews to add important context to quantitative outcome data. Importantly, we will look at the demographics of patients who participate versus those who decline. We will seek information from those who decline to explore ways in which these barriers to participation can be overcome.
The secondary objective is to determine whether participation in the intervention leads to changes in outcomes including physical activity, diet, body weight, HbA1c, liver enzymes, liver stiffness, blood pressure, lipid profile (all measured as part of standard care using the NAFLD Care Bundle) [18] and patient activation/empowerment. These preliminary data will provide some indication of whether the intervention could be effective as part of routine clinical care.
Methods
Study design
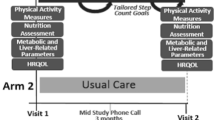
This feasibility study will use a single-centre (Newcastle upon Tyne Hospitals NHS Foundation Trust (NuTH)), one-arm, prospective design. Feasibility and acceptability of recruitment, the intervention (VITALISE), and associated outcome measures will be assessed using a mixed-methods approach. Health-related outcomes will be quantitatively assessed at baseline and 6-months. An interim measure of self-reported weight, physical activity and self-efficacy (via the Patient Activation Measure (PAM)) will be recorded at 12-weeks.
Sample size
With reference to best practice guidelines on sample size requirements for feasibility studies [19] and practical time frames, we will aim to recruit 35 patients over a 6-month time period.
Target population
Adults with newly diagnosed NAFLD (see inclusion criteria for definition).
Patient identification and consent process
Patients will be recruited from the Liver Unit at NuTH. Eligible patients will be given/sent a study invitation letter and participation information sheet. At the clinic visit, the treating physician will discuss the study with eligible patients following receipt of standard NHS care (i.e. review, examination, and blood tests). If the patient is interested in taking part, a research team member will provide them with more information about the study, answer any questions, obtain written informed consent, collect baseline data, and offer an induction to the intervention (either on the same day as the clinic appointment, or on an alternative day if preferred by the patient).
Inclusion criteria
-
• Aged over 18 years
-
• Clinical diagnosis of NAFLD following review by a hepatologist within the last 6-months
-
• Willing and able to provide written informed consent
Exclusion criteria
-
• Decompensated NASH cirrhosis (Child Pugh score ≥ 7)
-
• Diagnosed/previous eating disorder or purging as recorded in the medical notes
-
• Potentially harmful alcohol consumption (>14 units/week for females; >21 units/week for males)
-
• Known cancer (except skin cancer)
-
• Myocardial infarction within 6 months or uncontrolled cardiovascular disease
-
• Pregnant/considering pregnancy
-
• Inability to read and converse in English
Preparation procedures
A member of the research team will use a study-specific medical questionnaire to collect sociodemographic and clinical data including information on medical history and current medications (See supplementary file). Body weight, height, waist and hip circumference, blood pressure, blood tests (glucose, lipid profile, liver blood tests [LBTs], full blood count [FBC], HbA1c), liver stiffness measurement [LSM] by Fibroscan and calculation of clinical scores (QRISK3, Fibrosis-4 (FIB-4) Score, NAFLD Fibrosis Score (NFS)) will be measured as part of standard care. Patients with Type 2 diabetes (T2DM) will be monitored as part of standard care and medications adjusted in response to weight loss/dietary change/physical activity as necessary. This will be reported as part of the evaluation.
Patients will be asked to complete a physical activity questionnaire (the Godin leisure-time physical activity questionnaire [20]) and Patient Activation Measure (PAM) [21] as part of the research study at baseline, 12-weeks and 6-month follow-up. Patients will also be given an accelerometer to wear for 7-days (to objectively measure physical activity levels) which will be returned to the research team in a pre-paid envelope at baseline and 6-month follow-up.
VITALISE has been developed in collaboration with a commercial organisation, ‘Changing Health Limited’, who are a leading provider of lifestyle behaviour change programmes to the NHS (Changing Health | Digital Behaviour Change At Scale). Changing Health provide evidence-based education and lifestyle coaching digital solutions for people with type 2 diabetes across the UK and renders its innovative services customised to their clients’ requirements. With the patient’s permission, the research team will send their email address, and full name to Changing Health who will subsequently email the patient a welcome email allowing them to access the VITALISE intervention and setup their profile. A researcher will provide patients with a demonstration of how to login to VITALISE. Table 1 outlines the summary schedule of investigations.
Intervention
Patients will be given a personal login for VITALISE and will have continuous access for a 6-month period prior to their follow-up appointment with a hepatologist. They will be supported by monthly personalised tele-coaching sessions delivered by specifically trained lifestyle coaches. A pedometer will be provided to allow patients to record daily step count. Table 2 provides and overview of VITALISE content.
VITALISE lifestyle coaching
As part of the VITALISE intervention, patients will have access to monthly personalised tele-coaching sessions delivered by trained lifestyle coaches. Coaches will have received training on what NAFLD is, how it is investigated, different treatment options (aligned with clinical guidelines) and the evidence supporting lifestyle intervention for the management of NAFLD in accordance with clinical guidelines. Coaches are also trained in the use of brief motivational techniques and will have an in-depth knowledge of the information content and self-regulation tools provided to enable signposting of patients during coaching sessions.
The initial coaching appointment will last approximately 20 min and subsequent appointments 10–15 min. Coaching duration and frequency were informed by findings from previously studies [15, 22]. The coaches will facilitate person-centred behavioural goal setting and encourage self-monitoring of lifestyle behaviours by using the tracking tools embedded within VITALISE.
Additional support
A member of the research team will telephone each patient at the end of 12 weeks to answer any questions, promote continued engagement, and record any adverse events. Patients will be asked to self-report their weight, physical activity levels (step count and questionnaire) and complete the PAM.
Email reminders will be sent out to each patient once each week for the first four weeks, then monthly thereafter to prompt continued engagement with the intervention. Patients will have the opportunity to contact a member of the research team via telephone or email for assistance if they experience any technical difficulties during the study.
Outcomes and criteria for success
Criteria to judge the feasibility of progressing to a larger scale evaluation:
Recruitment rate
Six patients recruited per month over a 6-month time period.
Intervention uptake and engagement
≥ 80% of patients recruited logging in to the intervention.
Completion and follow-up rate
≥ 70% of those accessing the intervention providing data at 6-months follow-up.
These criteria aim to retain a sufficient sample size to determine acceptability and feasibility.
Primary outcome measures
In line with NIHR guidance on feasibility studies, the focus will be to estimate important parameters that are needed to inform the design of a subsequent larger evaluation, if appropriate. Table 3 details the feasibility study parameters.
We will collect demographic data (age, sex, ethnicity, socio-economic status) on patients who decline to take part and record reasons for declining/unsuitability to inform the way in which the programme is promoted in the future.
Acceptability of VITALISE will be assessed qualitatively using in-depth, semi-structured interviews and all patients recruited will be invited to take part. Topics will focus on patients’ perceived expectations, benefits, motivations, and barriers to using/accessing VITALISE. These topics will be explored in relation to age and liver disease severity. We will qualitatively assess fidelity of receipt and enactment of the intervention to provide an indication of whether participants used the intervention as intended to make behavioural changes. Our interview topic guide will contain specific questions about each component of the intervention to determine whether they were received by participants and whether they utilised each component as intended. This information will inform optimisation of the intervention ahead of a large-scale evaluation, if appropriate. Those who drop out of the intervention will be invited to interview to explore reasons for drop-out. Findings will inform intervention optimisation.
We will also collect data on the number of patients who log in to the intervention, the number of times they access each module, attend coaching appointments, and the length of coaching calls, to enable us to provide context around study outcomes – i.e., is it likely that any preliminary changes observed are a consequence of engagement with the intervention.
Secondary outcome measures
Body weight, height, waist and hip circumference, blood pressure, blood tests (glucose, lipid profile, LBTs, FBC, HbA1c), liver stiffness measurement and calculation of clinical scores (QRISK3, FIB-4, NFS) will be measured as part of standard care.
Physical activity
Physical activity will be objectively measured using an ActiGraph GT9X accelerometer worn on the non-dominant wrist for a minimum of 8 h per day over seven consecutive days during the first and last week of the intervention. Daily total activity counts, steps, time spent sedentary, and time spent in light, moderate, and vigorous activity will be recorded. Freedson thresholds based on metabolic equivalents will be used to demarcate to intensity of physical activity: sedentary (≤ 100 counts/min), light (101–1951 counts/min), moderate (1952–5724 counts/min), and vigorous activity (≥ 5725 counts/min) [23, 24]. Self-reported physical activity will be assessed with the Godin Leisure-Time Exercise Questionnaire using the total leisure activity score [20] at baseline, 12-weeks and 6-months.
Patient activation/empowerment
Patients will be asked to complete Patient Activation Measure (PAM) [21] at baseline, 12-weeks and 6-months. The PAM questionnaire collects data on an individual’s knowledge, skills and confidence to manage their health and is a global measure of an individual’s overall ability to manage their health.
Questionnaires will be given to patients in the clinic to take home and return by post in pre-paid envelopes alongside their accelerometer. We will look at return rates for questionnaires/accelerometers as part of the feasibility study and address issues/seek patient feedback on ways to improve this for the larger scale evaluation as necessary.
Safety reporting
This is a small-scale feasibility study, and therefore a formal data monitoring committee will not be convened. We do not anticipate any major risks or adverse events to arise as a result of taking part in this study. If an adverse event does occur, it will be recorded and categorised in terms of relatedness and severity in line with the CTCAE criteria. The research team will meet bi-weekly to monitor interim safety/feasibility data by reviewing recruitment/adherence rates and adverse events. The NuTH NHS Trust will be informed according to trust policy.
Data and statistical analysis
Quantitative analysis
The flow of participants throughout the trial will be reported in a Consolidated Standards of Reporting Trials (CONSORT) flowchart. Reasons for declining to take part in and for withdrawing from the study will also be recorded in the CONSORT flowchart. Descriptive statistics will be used to present baseline characteristics and feasibility outcomes. A paired t-test or Wilcoxon signed-rank test (depending on data distribution) will be used to evaluate changes in outcomes from pre- to post-intervention, with the mean difference and 95% confidence interval presented.
Qualitative analysis
Interviews will be audio recorded, transcribed verbatim, and analysed using thematic analysis [25]. All transcripts will be coded independently by one member of the research team, with a proportion (~ 50%) independently coded by a second research team member. Codes and potential themes will be discussed and agreed at the research team level and agreed for reporting purposes.
Research ethics approval
An independent NHS Research Ethics Committee (North East—Tyne & Wear South Research Ethics Committee) has provided a favourable ethical opinion for this study to commence (IRAS ID: 313,662).
Patient and public involvement
We have consulted extensively with patients with NAFLD to identify their supported self-management needs and preferences. A consistent finding is their lack of knowledge about what NAFLD is, how it progresses (and that it can progress) and current management options (“the only thing they said was to try and sort of lose a bit of weight…apart from that I’ve never ever had any advice or anything else”). Most patients we consulted did not understand the links between their lifestyle behaviours and NAFLD, including its progression. A lack of knowledge that NAFLD can be managed by making lifestyle changes was evident. This is likely due to the lack of NAFLD-specific resources available to facilitate communication with patients (“I couldn’t really go into it. It was so brief, what I got off my GP. She did tell me I could Google it and read up about it…But I haven’t”).
The findings from a series of interviews and workshops with patients have led to the development of VITALISE. The involvement of specifically trained lifestyle coaches was considered an essential intervention component to promote ongoing engagement and help patients overcome barriers to achieving clinically and personally meaningful goals.
Patients who assessed usability of VITALISE reported feeling empowered, informed and motivated to make lifestyle changes.
Modification of the protocol
Any modifications to the protocol will be agreed by the research team and study sponsor, and approved by an independent NHS Research Ethics Committee.
Discussion
Lifestyle intervention is recommended by national and international clinical guidelines for the management of NAFLD, regardless of disease severity [8,9,10]. However, clinicians struggle to support patients with NAFLD to make and sustain lifestyle changes due to a lack of NAFLD-specific interventions and tools to use in clinical practice. People with NAFLD find weight loss and weight loss maintenance a significant challenge and report a lack of NAFLD-specific, tailored support from HCPs [11, 12].
VITALISE is an evidence-informed intervention comprising of structured education and self-regulation tools to help support changes in diet and physical activity behaviours to initiate and maintain weight loss. VITALISE provides credible information for patients relating to what NAFLD is (and isn’t), and how it links to their lifestyle behaviours.
VITALISE offers patients the opportunity to access tailored dietary and physical activity support in their own time, outside of the hospital setting. This may remove some of the documented barriers to participation if face-to-face attendance at appointments is not required. Despite its potential, there are uncertainties relating to the feasibility and acceptability of using digital interventions to support clinical care in people living with NAFLD.
This mixed-methods study will address uncertainties relating to the feasibility and acceptability of delivering online, behaviour change interventions to patients with NAFLD. The findings will inform optimisation of the intervention, if required, and the assessment of outcomes will provide information on the likely size and variability of intervention effects. Collectively, the data generated will inform the design of a subsequent, adequately-powered, larger scale evaluation of VITALISE, if appropriate.
Availability of data and materials
This manuscript does not contain any data. Supporting material is available as supplementary information files.
Abbreviations
- NAFLD:
-
Non-alcoholic fatty liver disease
- VITALISE:
-
InterVention to promote lIfesTyle change in non-Alcoholic fatty LIver diseaSE
- NASH:
-
Non-alcoholic steatohepatitis
- HCP:
-
Healthcare professional
- NuTH:
-
Newcastle upon Tyne Hospitals NHS Foundation Trust
- PAM:
-
Patient activation measure
- LBT:
-
Liver blood tests
- FBC:
-
Full blood count
- LSM:
-
Liver stiffness measurement
- FIB-4:
-
Fibrosis-4 score
- NFS:
-
NAFLD fibrosis score
- T2DM:
-
Type 2 diabetes
- CONSORT:
-
Consolidated standards of reporting trials
References
Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67(1):123–33.
Williams R, Alexander G, Aspinall R, Bosanquet J, Camps-Walsh G, Cramp M, et al. New metrics for the lancet standing commission on liver disease in the UK. The Lancet. 2017;389(10083):2053–80.
McPherson S, Hardy T, Henderson E, Burt AD, Day CP, Anstee QM. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: Implications for prognosis and clinical management. J Hepatol. 2015;62(5):1148–55.
Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol. 2013;10:330–44.
Stefan N, Häring H-U, Cusi K. Non-alcoholic fatty liver disease: causes, diagnosis, cardiometabolic consequences, and treatment strategies. Lancet Diabetes Endocrinol. 2019;7(4):313–24.
Hallsworth K, Adams L. Lifestyle interventions in NAFLD/NASH: Facts and figures. J Hep Reports. 2019;1:468–79.
Romero-Gómez M, Zelber-Sagi S, Trenell M. Treatment of NAFLD with diet, physical activity and exercise. J Hepatol. 2017;67(4):829–46.
Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American association for the study of liver diseases. Hepatology. 2018;67(1):328–57.
European Association for the Study of the Liver, European Association for the Study of Diabetes, European Association for the Study of Obesity. EASL–EASD–EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64(6):1388–402.
NICE. National Institute for Clinical Excellence (NICE) Guidelines: Non-alcoholic fatty liver disease (NAFLD): assessment and management (NG49) 2016.
Avery L, Exley C, McPherson S, Trenell MI, Anstee QM, Hallsworth K. Lifestyle behavior change in patients with nonalcoholic fatty liver disease: a qualitative study of clinical practice. Clin Gastroenterol Hepatol. 1968;2017:15.
Hallsworth K, Dombrowski SU, McPherson S, Anstee QM, Avery L. Using the theoretical domains framework to identify barriers and enabling factors to implementation of guidance for the diagnosis and management of nonalcoholic fatty liver disease: a qualitative study. Transl Behav Med. 2019;10:1016.
Tincopa MA, Wong J, Fetters M, Lok AS. Patient disease knowledge, attitudes and behaviours related to non-alcoholic fatty liver disease: a qualitative study. BMJ Open Gastroenterol. 2021;8(1):e000634.
Bartholomew LK, Parcel GS, Kok G, Gottlieb NH, Fernández M. Planning health promotion programs: An Intervention Mapping approach: San Francisco. CA: Jossey-Bass; 2011.
Hallsworth K, McPherson S, Anstee QM, Flynn D, Haigh L, Avery L. Digital Intervention with lifestyle coach support to target dietary and physical activity behaviors of adults with nonalcoholic fatty liver disease: systematic development process of VITALISE using intervention mapping. J Med Internet Res. 2021;23(1):e20491.
Eldridge SM, Lancaster GA, Campbell MJ, Thabane L, Hopewell S, Coleman CL, et al. Defining feasibility and pilot studies in preparation for randomised controlled trials: development of a conceptual framework. PLoS ONE. 2016;11(3): e0150205.
Eldridge SM, Chan CL, Campbell MJ, Bond CM, Hopewell S, Thabane L, et al. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. BMJ : Brit Med J. 2016;24(355): i5239.
Neilson LJ, Macdougall L, Lee PS, Hardy T, Beaton D, Chandrapalan S, et al. Implementation of a care bundle improves the management of patients with non-alcoholic fatty liver disease. Frontline Gastroenterol. 2021;12(7):578–85.
Teare MD, Dimairo M, Shephard N, Hayman A, Whitehead A, Walters SJ. Sample size requirements to estimate key design parameters from external pilot randomised controlled trials: a simulation study. Trials. 2014;15:264.
Amireault S, Godin G, Lacombe J, Sabiston CM. The use of the Godin-Shephard leisure-time physical activity questionnaire in oncology research: a systematic review. BMC Med Res Methodol. 2015;15:60.
Hibbard JH, Stockard J, Mahoney ER, Tusler M. Development of the Patient Activation Measure (PAM): conceptualizing and measuring activation in patients and consumers. Health Serv Res. 2004;39(4 Pt 1):1005–26.
Little P, Stuart B, Hobbs FDR, Kelly J, Smith E, Bradbury K, et al. Randomised controlled trial and economic analysis of an internet-based weight management programme: POWeR+ (Positive Online Weight Reduction). Health Technol Assess. 2017;21(4):1–62.
Leinonen AM, Ahola R, Kulmala J, Hakonen H, Vähä-Ypyä H, Auvinen J, et al. Measuring physical activity in free-living conditions—comparison of three accelerometry-based methods. Front Physiol. 2017;7:681.
Freedson PS, Melanson E, Sirard J. Calibration of the computer science and applications Inc accelerometer. Med Sci Sports Exerc. 1998;30:777–81.
Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3(2):77–101.
Funding
This work is supported by a Newcastle upon Tyne Hospitals NHS Charity Joint Research Executive Scientific Committee project grant. The funding source had no role in the design of this study and will not have any role during its execution, analyses, interpretation of the data, or decision to submit results.
Author information
Authors and Affiliations
Contributions
LA, SMc and KH conceived the study and are grant holders. LA, HS, SMc and KH developed the study design and intervention, and were responsible for writing the first draft of the study protocol. SMc and KH provided clinical oversight and expertise. LA provided health psychology and qualitative research expertise and is leading the qualitative analysis of patient interviews and fidelity assessment. HS is a PhD student leading the study at site. All authors contributed to refinement of the study protocol and approved the final submitted manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
An independent NHS Research Ethics Committee (North East—Tyne & Wear South Research Ethics Committee) has ethically approved the study (IRAS ID: 313662). Patients will provide written informed consent prior to participating in this research.
Consent for publication
Not applicable.
Competing interests
The authors have no conflicts of interest to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Avery, L., Smith, H., McPherson, S. et al. Feasibility and acceptability of an evidence-informed digital intervention to support self-management in people with non-alcoholic fatty liver disease: protocol for a non-randomised feasibility study (VITALISE). Pilot Feasibility Stud 9, 62 (2023). https://doi.org/10.1186/s40814-023-01286-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40814-023-01286-2