Abstract
Purpose
Benign prostatic hyperplasia (BPH) commonly impacts the quality of life in older men. However, there is lack of research on relationship between dietary niacin intake and the risk of BPH. The purpose of this study was to investigate the relationship between dietary niacin intake and the risk of BPH.
Methods
Data from the NHANES spanning 2003 to 2008 were utilized. BPH was determined using a self-report questionnaire, while dietary niacin intake was calculated based on the mean of two distinct diet interviews. Multivariate logistic regressions were performed to explore the association, supplemented with restricted cubic splines and subgroup analysis.
Results
A total of 700 males were enrolled, of which 653 men had BPH. After adjusting for all covariates, a high dietary intake of niacin was associated with an increased risk of BPH (OR: 1.04; 95%CI: 1.01–1.07). Furthermore, when the lowest dietary niacin intake is used as the reference, the highest tertile is associated with an increased risk of BPH (OR: 2.34, 95% CI: 1.24-4,42). Restricted cubic splines demonstrated a positive correlation between dietary niacin intake and BPH risk.
Conclusions
The study results demonstrated a positive association between dietary niacin intake and the risk of BPH in elderly men in the US. These findings underscore the importance of systematic assessment before supplementing micronutrients in elderly men.
Similar content being viewed by others
Introduction
Benign prostatic hyperplasia (BPH), also known simply as prostatic hyperplasia, is a non-neoplastic condition marked by enlargement of the prostate gland [1]. It is a very common occurrence among older men, accompanied mainly by bothersome lower urinary tract symptoms (LUTS), including difficulty initiating urination, weak urine stream, and frequent nocturia [2]. In recent years, with the acceleration of global population aging, the incidence of BPH has sharply increased, posing a significant economic burden. According to the 2019 Global Burden of Disease statistics, the prevalence of BPH has increased by 105.7% since 1990, with the fastest growth rate observed in the older men between the age of 65 and 69 years [3]. Despite numerous investigations, the exact mechanism of BPH remains poorly understood. Several potential risk factors contributing to BPH have been identified, such as age, obesity, diabetes, hypertension, smoking, alcohol use, and nutritional factors [4,5,6]. Notably, nutritional factors are among the easier ones to modify in order to control the onset and progression of BPH [7]. Consequently, it has become an important research direction in BPH to identify effective preventive and control strategies.
Niacin, also known as nicotinic acid or Vitamin B3, is the precursor to nicotinamide adenine dinucleotide (NAD) and NAD phosphate (NADP), both essential for energy metabolism and redox reactions [8]. Currently, niacin is primarily used as a lipid-lowering drug to treat atherosclerotic cardiovascular disease [9, 10], by regulating lipid metabolism, enhancing vascular endothelial function, and exhibiting antioxidant and anti-inflammatory properties [11]. These functions make it a popular supplement among middle-aged and older adults. However, Holton et al. found no association between dietary micronutrients and the alleviation of LUTS [12]. Furthermore, recent large-scale population-based studies showed that daily supplementation with folic acid, retinyl esters, and carotenoids may actually increase the risk of developing BPH [7, 13]. These studies indicate that the slower metabolism in middle-aged and older adults can lead to an accumulation of supplemented micronutrients, potentially increasing the proliferative capacity of prostate cells and elevating the risk of BPH. This reminds us that comprehensive evaluation is necessary for dietary micronutrients supplementation in middle-aged and older adults, as improving one aspect may exacerbate others. Therefore, exploring the relationship between dietary niacin intake and the risk of BPH becomes increasingly urgent.
The National Health and Nutrition Examination Survey (NHANES), dedicated to evaluating the impacts of nutritional status on health promotion and disease prevention in the United States, furnishes us with a strong platform and extensive data to thoroughly investigate the relationship between dietary niacin intake and the risk of prostatic hyperplasia. Weighted multivariable logistic regression analysis was conducted on NHANES 2003–2008 data to explore the relationship between dietary niacin intake and the risk of BPH, complemented by subgroup analysis and restricted cubic splines (RCSs).
Materials and methods
Study design and subjects
The NHANES, administered by the National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention, is an ongoing program designed to assess the health and nutritional status of the non-institutionalized civilian population in the US. A sophisticated multistage probability sampling design ensures an accurate representation of the nutritional and health status of the US population in sample selection. Data collection comprises demographics, dietary, examination, laboratory, and questionnaire information obtained through personal interviews and clinical examinations on a biennial basis. The National Center for Health Statistics Institutional Review Board (NCHS IRB/ERB) reviewed and approved the study protocols, and informed written consent was provided by all participants at enrollment. Therefore, a second review by our hospital’s ethics committee is unnecessary. Our study also aligned with The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
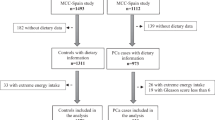
Prostate-related diseases, including BPH and prostate cancer, have been collected in NHANES since 2001 and ceased in 2008. Dietary-related data have been collected since 1999 in NHANES, but the collection of two-day dietary data began in 2003. The data from the 3-year cycles of NHANES 2003 to 2008 were utilized to explore the association between dietary niacin intake and the risk of BPH, where two assessments of dietary data provided increased accuracy. Based on the study objectives and data availability, a total of 30,619 participants from the NHANES 2003–2008 were initially considered as potentially eligible for our study. We then applied a series of inclusion and exclusion criteria to select the final sample for analysis. The study ultimately included men aged 40 and above who had completed the prostate enlargement, BPH, or prostate cancer questionnaire assessments, and who provided data on daily niacin intake. Participants diagnosed with prostate cancer or those lacking covariate information were excluded. Consequently, the final analysis sample comprised 700 participants. The exclusion criteria and the corresponding numbers of excluded participants are as follows: (1) Female participants: 15,473; (2) Participants younger than 40 years: 10,032; (3) Participants without BPH assessment data: 4,155 (3,469 without BPH data and 686 without prostate enlargement assessment data); (4) Participants diagnosed with prostate cancer: 130; (5) Participants lacking dietary niacin data: 68; (6) Participants lacking PIR (Poverty Income Ratio) data: 49, lacking BMI data: 11, and lacking alcohol use data: 1. The detailed sample selection process is illustrated in Fig. 1.
Assessment of BPH
In the Prostate condition questionnaires, three key questions pertain to prostate enlargement: KIQ121: “Have you ever been told by a doctor or health professional that you had an enlarged prostate gland?”, KIQ141: “Was it a benign enlargement - that is, not cancerous, also called benign prostatic hypertrophy?”, and KIQ182: “Was the enlargement due to cancer?”. When participants answer “No” to the first question, they are considered to have no BPH. When participants answer “Yes” to the first question, those who also answer “Yes” to the second question are considered to have BPH. However, participants who answer “Yes” to the third question are considered to have prostate cancer and are excluded from the study. To ensure the accuracy of participant diagnoses, those with unknown, refused, or missing answers are excluded [13, 14].
Measurement of dietary niacin intake
The “What We Eat in America” dietary interview component employed the standardized Automated Multiple-Pass Method (AMPM) through a collaboration between the US Department of Agriculture (USDA) and the US Department of Health and Human Services. The Food and Nutrient Database for Dietary Studies (FNDDS) provided the nutritional values for all items in the diet, detailing the nutritional profiles of each food reported in NHANES. This database converts food items reported in the 24-hour dietary recalls into nutrient values and is validated for use in nutritional research. For managing recipes, standard USDA recipes were used, and nutrient values for unique recipes were estimated using ingredient-level data from the FNDDS. More information can be found on the NHANES website: https://wwwn.cdc.gov/Nchs/Nhanes. Eligible NHANES participants underwent two 24-hour dietary recall interviews, where they detailed the types and quantities of foods consumed during two distinct 24-hour periods prior to each interview (from midnight to midnight). The first dietary recall was conducted face to face at the Mobile Examination Center, and the subsequent interview was completed via telephone approximately 3 to 10 days later. Dietary intake data were collected using the NHANES computer-assisted diet interview (CADI) system by trained interviewers. Dietary niacin intake was determined by averaging the results from two dietary interviews. Our study exclusively focused on niacin derived from food sources, excluding supplements.
Potential covariates
Based on previous research [7], multiple factors including age, race, education, marital status, poverty-income ratio (PIR), body mass index (BMI), smoking status, alcohol consumption, diabetes, hypertension, and cardiovascular disease (CVD) should be considered as covariates in analyzing the relationship between BPH and dietary niacin intake. Considering the exposure variable is diet-related, total energy intake of participants was also included as a covariate. In detail, the demographic characteristics include age, race, education, marital status, and PIR. Age was treated as a continuous variable and also converted into a binary variable (< 60y vs. ≥ 60y). Race was classified as Mexican American, Other Hispanic, Non-Hispanic White, Non-Hispanic Black, or other. Education level was categorized into three groups: less than high school, high school, and more than high school. Marital status included cohabitation or solitude. The PIR was categorized as follows: ≤1.3, 1.3–3.5, and ≥ 3.5. BMI was calculated using the standard formula: BMI = weight (kg) / height (m)2, and then categorized as follows: <25 kg/m2, 25–30 kg/m2, and ≥ 30 kg/m2. Participants’ smoking status was classified based on two questions: whether they had smoked fewer than 100 cigarettes in their lifetime, and if they were currently smoking. Participants who answered no to the first question were classified as non-smokers, those who answered yes to both questions were categorized as current smokers, and those who did not fit into either category were defined as former smokers. Alcohol consumption status was determined by participants’ response to the question “Do you drink at least 12 drinks per year?”, categorizing them as either “no” or “yes”. Participants labeled as diabetic either had a prior diabetes diagnosis or a fasting blood glucose level > 126 mg/dL. Participants were classified as having hypertension if they had received a diagnosis of hypertension, were prescribed medication for hypertension, or if their mean of multiple blood pressure measurements was ≥ 140/90 mmHg. Participants were classified as having a history of CVD if they experienced any of the following: coronary heart disease, congestive heart failure, heart attack, stroke, or angina.
Statistical analysis
Following NHANES statistical guidelines, appropriate sample weights were used to offset the complexities of the multi-stage sampling design and enhance data accuracy. The final weights for weighted analyses were calculated by multiplying 2 years of Mobile Examination Center (MEC) weights by 1/3. Continuous variables were presented as weighted mean ± standard error, while categorical variables were expressed as weighted proportions (%). To examine differences in variable characteristics across dietary niacin intake groups (Tertiles), chi-square tests for categorical variables and analysis of variance (ANOVA) for continuous variables were conducted respectively.
The association between dietary niacin intake and the risk of BPH was explored using three distinct logistic regression models. The odds ratio (OR) and 95% confidence intervals (CIs) were used to report the results of the corresponding regression analyses. No variables were adjusted in Model 1, also known as the crude model. In Model 2, also known as the minimally adjusted model, adjustments were made for age, race, education levels, PIR, and marital status. Model 3, referred to as the fully adjusted model, included adjustments for additional variables based on those accounted for in Model 2. Subsequently, logistic regression analyses were conducted again, after transforming dietary niacin intake into categorical variables by tertiles. For calculating the P-value for trend, the stratified median was treated as a quasi-continuous variable in the models. Moreover, weighted restricted cubic splines (RCSs) were utilized to elucidate the dose-response relationship between dietary niacin intake and the risk of BPH. The nonlinearity was evaluated through the likelihood ratio test. To explore potential differential associations between dietary niacin intake and the risk of BPH, participants were stratified by age, marital status, BMI, smoking status, alcohol status, and diagnosis of hypertension, DM, and CVD. During subgroup analysis, dietary niacin intake was considered as both a continuous and categorical variable, respectively. Interaction terms were used to test heterogeneity among subgroups, with P for interaction < 0.05 indicating significant heterogeneity.
All analyses were conducted using EmpowerStats software (www.empowerstats.com; X&Y solutions, Inc., Boston MA) and R software (R4.2.3; http://www.R-project.org; The R Foundation), with statistical significance set at a two-sided P-value < 0.05.
Results
Baseline characteristics of study population
After applying inclusion and exclusion criteria, the final analysis included 700 participants: 653 with BPH and 47 controls. The average age of the men analyzed was 64.39 ± 0.53 years, with the BPH and control groups having mean ages of 67.95 and 64.14 years, respectively. Compared to healthy participants, the BPH group had a higher dietary niacin intake, averaging 27.89 ± 0.49 mg versus 23.37 ± 1.33 mg. Table 1 describes the baseline characteristics of participants, grouped by tertiles of dietary niacin intake. The results indicate that compared to the lowest tertile of dietary niacin intake, participants in the highest tertile had a higher proportion of alcohol intake, increased risk of BPH, and a lower risk of CVD. In summary, the BPH group demonstrated higher dietary niacin intake, and higher dietary niacin intake was associated with an increased risk of BPH.
Association between dietary niacin intake and BPH
Multivariate logistic regression analysis was performed to explore the association between dietary niacin intake and BPH. As Table 2 demonstrates, considering dietary niacin intake as a continuous variable, higher intake was associated with an increased risk of BPH (OR: 1.04, 95%CI: 1.01, 1.07, P = 0.02), even after adjusting for all potential confounders. When dietary niacin intake is categorized into tertiles and taking the first quintile as the reference, the highest quintiles of dietary niacin intake were associated with higher odds for BPH across Model 1 (OR: 2.83, 95%CI: 1.29,6.23, P = 0.01), Model 2 (OR: 2.22, 95%CI: 1.16, 4.28, P = 0.02), and Model 3 (OR: 2.34, 95%CI: 1.24, 4.42, P = 0.01), with all P for trend values less than 0.05. The dose-response analysis using restricted cubic splines (RCS) showed that an increase in dietary niacin intake was associated with a higher risk of BPH (P for overall effect = 0.0269; P for nonlinearity = 0.453; Fig. 2).
Dose–response relationship analysis between dietary niacin intake and BPH, weighted. RCS regression was adjusted for age, race, marital status, education level, PIR, BMI, smoking status, alcohol consumption, diabetes mellitus, hypertension, and CVD (Model 3). The red solid line represents ORs, and red-shaded region represents 95% CI. BPH: Benign Prostatic Hyperplasia; CVD: cardiovascular disease; BMI: body mass index; PIR: poverty-to-income ratio; RCS: restricted cubic spline; OR: odds ratio; CI: confidence interval
Subgroup analysis
Subgroup analyses were conducted twice, treating dietary niacin intake as continuous and categorical variables, respectively. During the subgroup analysis, all potential covariates were adjusted for, except the stratified variables. As demonstrated in Table 3, participants older than 60 years still showed a statistically significant relationship between dietary niacin intake and a higher risk of BPH. Participants with a history of hypertension or CVD also demonstrated a relationship between dietary niacin intake and a higher risk of BPH (Fig. 3). The outcomes of all other subgroup analyses, focusing on marital status, BMI category, smoking and alcohol status, and history of diabetes, are shown in Fig. 3; Table 3, with no significant interactions observed.
Association between dietary niacin intake and BPH in different subgroups, weighted. Analyses were adjusted for age, race, marital status, education level, PIR, BMI, smoking status, alcohol consumption, diabetes mellitus, hypertension, and CVD (Model 3). OR: odds ratio; CI: confidence interval; BPH: Benign Prostatic Hyperplasia; BMI: body mass index; PIR: poverty-to-income ratio; CVD: cardiovascular disease
Discussion
Research exploring the relationship between dietary niacin intake and risk of BPH from the perspective of the elderly men is severely lacking. From this nationally representative cross-sectional study in US, the results reveal a pronounced dose-response correlation between elevated niacin consumption in the diet and the increased occurrence of BPH. Moreover, the results remained consistent when comparing the highest tertile of dietary niacin intake with the lowest tertile, even after adjusting for all covariates. Subgroup analysis revealed that individuals aged over 60 years, with a history of hypertension and CVD, exhibit an increased risk of developing BPH. Importantly, no significant interaction was detected among all analyzed subgroups.
Mechanistically, testosterone plays a crucial role in the development of benign prostatic hyperplasia. In animal models, timely testosterone supplementation to castrated rats promotes prostate hyperplasia [15]. However, a paradox exists wherein the prostate enlarges despite aging and a decrease in testosterone levels. Subsequent research has confirmed that dihydrotestosterone (DHT) in prostate tissue is a key factor in promoting BPH, which increases with age [16]. Moreover, some studies suggest that testosterone supplementation in elderly individuals can reduce the risk of BPH by decreasing local inflammation and oxidative stress [17, 18]. Hence, some studies suggest that metabolic syndrome (Mets) is one of the significant risk factors for BPH, inducing chronic inflammation and prostate proliferation [19, 20]. However, a recent Mendelian randomization study has provided genetic evidence suggesting that testosterone is the initiating factor for BPH, while metabolic syndrome mainly promotes BPH by altering testosterone levels [21]. On the contrary, uric acid, one of the substances associated with metabolic syndrome, is believed to potentially reduce the risk of BPH [22]. Another Korean study involving 101,091 individuals indicated that higher blood uric acid levels were associated with a lower likelihood of lower urinary tract symptoms (LUTS), as per recent findings [23]. These findings suggest that the pathogenesis of BPH is currently inconsistent and extremely complex. Therefore, improving BPH pharmacologically requires extreme caution, especially when considering the use of certain dietary micronutrients.
Numerous studies have explored insulin resistance as a contributing factor to BPH development [24,25,26]. In vitro studies have found that insulin has a direct mitogenic effect on prostate epithelial cells [27]. Another study found a significant correlation between fasting plasma insulin levels and prostate volume, with the annual prostate growth rate being significantly faster in the top quartile group for serum insulin levels compared to the bottom quartile group [28]. Chen et al. found that in elderly patients newly diagnosed with type 2 diabetes, plasma viscosity increases with rising blood glucose levels and body mass index [29]. Previous research has suggested that niacin intake may impair insulin sensitivity and increase insulin levels by affecting the expression of microRNAs [30]. Therefore, it is reasonable to hypothesize that dietary intake of niacin may reduce insulin sensitivity in adipose tissue, thereby promoting insulin secretion and potentially leading to prostatic hyperplasia.
Additionally, increased testosterone secretion may also contribute to the link between niacin intake and a higher risk of benign prostatic hyperplasia (BPH). A clinical study found that niacin intake may increase testosterone levels in the body [31]. Another study using rats confirmed that niacin improves testicular structure through its antioxidant effects, thereby enhancing testosterone secretion [32, 33]. These studies indicate that while supplementing with micronutrients can improve the function of most organs that decline with aging, it may also accelerate prostatic hyperplasia. Therefore, it is particularly important to supplement micronutrients appropriately and in a personalized manner. A crucial point is that the metabolism of micronutrients like niacin differs in elderly individuals compared to adults. A study on folate metabolism in vitamin B12 showed that both the folic acid content in the blood and red blood cells (RBCs) increases in older American men, regardless of whether they use supplements containing folic acid, which suggests that folate metabolism may be influenced by aging [34]. This also implies that increased RBC folate levels could promote the development of BPH, necessitating careful consideration of folate supplementation in the elderly [13]. Similarly, when elderly individuals consume niacin that they cannot metabolize effectively, it leads to the accumulation of niacin and its metabolites. The former can directly promote cell replication and survival, while the latter may induce chronic inflammation, together facilitating the development of BPH [35]. In summary, a systematic assessment of health and cautious use of micronutrients are crucial for the elderly.
As mentioned earlier, niacin plays a significant role in cardiovascular disease prevention, but it may also pose health risks [36]. A preliminary study suggests that niacin metabolites may increase the risk of cardiovascular disease by triggering vascular inflammation [35]. Furthermore, another study indicates that niacin therapy may increase the risk of developing new-onset diabetes [37]. They speculate that the increase in niacin intake may be due to increased intake of red meat, leading to the occurrence of obesity, which is closely associated with the development of diabetes [38]. The adverse effects of niacin supplementation on the prostate are rarely mentioned or studied. However, there are studies beginning to focus on the potential association between retinyl esters and total carotenoids and a higher risk of BPH [7]. This suggests that supplementation of such micronutrients in older adults should be individualized, and timely consultation with a doctor or dietitian to choose appropriate alternatives is essential. Certainly, the molecular mechanisms underlying the impact of dietary niacin intake on the prostate remain unclear and warrant exploration through numerous in vivo and in vitro experiments.
Study strengths and limitations
Our study has several strengths that deserve clinical attention. To our knowledge, this is the first study to investigate the relationship between dietary niacin intake and the risk of BPH. The use of the NHANES database ensures data accuracy and allows for the adjustment of various covariates. Additionally, we conducted subgroup and dose-response relationship analyses, which further ensure the reliability of our conclusions. Considering the unique nature of the prostate gland compared to other organs such as the heart, liver, and kidneys, our study provides valuable insights into the potential benefits and disadvantages of dietary supplementation, particularly in older adults. Undoubtedly, every study has its limitations deserving acknowledgment. Firstly, the data currently utilized in the study is outdated, based on survey conducted 20 years ago, limiting the applicability to the present population. Secondly, the study solely relied on dietary niacin intake to investigate its association with BPH, without assessing serum or blood levels of niacin in humans. Moreover, due to the inherent limitations of cross-sectional studies, causal relationship between niacin intake and risk of BPH cannot be derived. Thirdly, the BPH diagnosis in NHANES predominantly relies on a questionnaire format without other objective assessments, which not only diminishes diagnostic accuracy but also compromises the reliability of the results. The same issue exists for the dietary niacin data, as self-reported dietary data may be subject to recall bias. Consequently, more large-scale studies are necessary in the future to further confirm our findings and provide more evidence for clinical applications.
Conclusion
Our findings indicate that higher dietary niacin intake may potentially exacerbate BPH in older men. When considering clinical practice, it is important to assess prostate health before recommending nutritional supplements for middle-aged and older men to ensure that the treatment of one condition does not worsen another. For older men with BPH, clinicians should consider reducing or avoiding the use of niacin and its related compounds. Given the inherent limitations of our study, further well-designed research is essential to validate and expand upon our conclusions.
Data availability
The study data are available on the NHANES website (https://www.cdc.gov/nchs/nhanes/index.htm). More detailed analysis data and corresponding R code can be provided upon reasonable request by contacting the corresponding author.
References
Berry SJ, Coffey DS, Walsh PC, Ewing LL. The development of human benign prostatic hyperplasia with age. J Urol. 1984;132(3):474–9.
Lu Y, Fan S, Song Y, Liu K, Zhou K, Kang J, Wang S, Yang Y, Liu X. The association between anogenital distance and benign prostatic hyperplasia related lower urinary tract symptoms in Chinese aging men. World J Urol. 2021;39(7):2645–53.
Zhu C, Wang DQ, Zi H, Huang Q, Gu JM, Li LY, Guo XP, Li F, Fang C, Li XD, et al. Epidemiological trends of urinary tract infections, urolithiasis and benign prostatic hyperplasia in 203 countries and territories from 1990 to 2019. Mil Med Res. 2021;8(1):64.
Xia BW, Zhao SC, Chen ZP, Chen C, Liu TS, Yang F, Yan Y. The underlying mechanism of metabolic syndrome on benign prostatic hyperplasia and prostate volume. Prostate. 2020;80(6):481–90.
Suzuki S, Platz EA, Kawachi I, Willett WC, Giovannucci E. Intakes of energy and macronutrients and the risk of benign prostatic hyperplasia. Am J Clin Nutr. 2002;75(4):689–97.
Parsons JK, Im R. Alcohol consumption is associated with a decreased risk of benign prostatic hyperplasia. J Urol. 2009;182(4):1463–8.
Zhou H, Xu M, Pan Y, Wang S, Xu Z, Liu L, Liu X. The association between several serum micronutrients and benign prostatic hyperplasia: results from NHANES 2003–2006. Prostate. 2024;84(2):212–20.
Kirkland JB, Meyer-Ficca ML. Niacin. Adv Food Nutr Res. 2018;83:83–149.
Ganji SH, Kamanna VS, Kashyap ML. Niacin and cholesterol: role in cardiovascular disease (review). J Nutr Biochem. 2003;14(6):298–305.
McKenney J. New perspectives on the use of niacin in the treatment of lipid disorders. Arch Intern Med. 2004;164(7):697–705.
Meyers CD, Kamanna VS, Kashyap ML. Niacin therapy in atherosclerosis. Curr Opin Lipidol. 2004;15(6):659–65.
Holton KF, Marshall LM, Shannon J, Lapidus JA, Shikany JM, Bauer DC, Barrett-Connor E, Parsons JK. Dietary antioxidants and longitudinal changes in lower urinary tract symptoms in Elderly men: the osteoporotic fractures in men study. Eur Urol Focus. 2016;2(3):310–8.
Chen T, Huang Y. Red blood cell folate and benign prostatic hyperplasia: results from the NHANES 2001–2008. Aging Male. 2024;27(1):2336625.
Yang L, Liu Z, Peng Z, Song P, Zhou J, Wang L, Chen J, Dong Q. Exposure to Di-2-ethylhexyl phthalate and Benign Prostatic Hyperplasia, NHANES 2001–2008. Front Endocrinol (Lausanne). 2021;12:804457.
Nishi N, Oya H, Matsumoto K, Nakamura T, Miyanaka H, Wada F. Changes in gene expression of growth factors and their receptors during castration-induced involution and androgen-induced regrowth of rat prostates. Prostate. 1996;28(3):139–52.
Chughtai B, Forde JC, Thomas DD, Laor L, Hossack T, Woo HH, Te AE, Kaplan SA. Benign prostatic hyperplasia. Nat Rev Dis Primers. 2016;2:16031.
Pearl JA, Berhanu D, François N, Masson P, Zargaroff S, Cashy J, McVary KT. Testosterone supplementation does not worsen lower urinary tract symptoms. J Urol. 2013;190(5):1828–33.
Vignozzi L, Cellai I, Santi R, Lombardelli L, Morelli A, Comeglio P, Filippi S, Logiodice F, Carini M, Nesi G, et al. Antiinflammatory effect of androgen receptor activation in human benign prostatic hyperplasia cells. J Endocrinol. 2012;214(1):31–43.
Vignozzi L, Gacci M, Maggi M. Lower urinary tract symptoms, benign prostatic hyperplasia and metabolic syndrome. Nat Rev Urol. 2016;13(2):108–19.
Lotti F, Corona G, Vignozzi L, Rossi M, Maseroli E, Cipriani S, Gacci M, Forti G, Maggi M. Metabolic syndrome and prostate abnormalities in male subjects of infertile couples. Asian J Androl. 2014;16(2):295–304.
Lin L, Wang W, Xiao K, Guo X, Zhou L. Genetically elevated bioavailable testosterone level was associated with the occurrence of benign prostatic hyperplasia. J Endocrinol Invest. 2023;46(10):2095–102.
Zhou H, Xu M, Hao X, Xu Z, Pan Y, Liu X. Association of serum uric acid levels with benign prostatic hyperplasia in US men: results from NHANES 2005–2008. Aging Male. 2023;26(1):2275775.
Hwang J, Ryu S, Ahn JK. Higher levels of serum uric acid have a Significant Association with lower incidence of lower urinary tract symptoms in healthy Korean men. Metabolites 2022, 12(7).
Zhao S, Wang Y, Wu W, Yang S, Feng L, Tao F, Ge W, Shen M, Xu W. Nonalcoholic fatty liver disease and risk of prostatic diseases: roles of insulin resistance. Andrologia. 2021;53(6):e14060.
Breyer BN, Sarma AV. Hyperglycemia and insulin resistance and the risk of BPH/LUTS: an update of recent literature. Curr Urol Rep. 2014;15(12):462.
Vikram A, Jena G, Ramarao P. Insulin-resistance and benign prostatic hyperplasia: the connection. Eur J Pharmacol. 2010;641(2–3):75–81.
McKeehan WL, Adams PS, Rosser MP. Direct mitogenic effects of insulin, epidermal growth factor, glucocorticoid, cholera toxin, unknown pituitary factors and possibly prolactin, but not androgen, on normal rat prostate epithelial cells in serum-free, primary cell culture. Cancer Res. 1984;44(5):1998–2010.
Hammarsten J, Högstedt B. Hyperinsulinaemia as a risk factor for developing benign prostatic hyperplasia. Eur Urol. 2001;39(2):151–8.
Chen Z, Miao L, Gao X, Wang G, Xu Y. Effect of obesity and hyperglycemia on benign prostatic hyperplasia in elderly patients with newly diagnosed type 2 diabetes. Int J Clin Exp Med. 2015;8(7):11289–94.
Montastier E, Beuzelin D, Martins F, Mir L, Marqués MA, Thalamas C, Iacovoni J, Langin D, Viguerie N. Niacin induces mir-502-3p expression which impairs insulin sensitivity in human adipocytes. Int J Obes (Lond). 2019;43(7):1485–90.
Hestiantoro A, Astuti BPK, Joyo EO, Febri RR, Silvana V, Muharam R. Vitamin B(3) (niacin), B(6), C, and iron intake are associated with the free androgen index, especially in normoandrogenic polycystic ovary syndrome. J Turk Ger Gynecol Assoc. 2022;23(3):130–6.
Shomali T, Taherianfard M, Dalvand M, Namazi F. Effect of pharmacological doses of niacin on testicular structure and function in normal and diabetic rats. Andrologia. 2018;50(10):e13142.
Azimi Zangabad E, Shomali T, Roshangar L. Effects of pharmacological doses of niacin on subacute glucocorticoid-induced testicular damage in rats. Pharmacol Res Perspect. 2023;11(5):e01128.
Rycyna KJ, Bacich DJ, O’Keefe DS. Divergence between dietary folate intake and concentrations in the serum and red blood cells of aging males in the United States. Clin Nutr. 2016;35(4):928–34.
Ferrell M, Wang Z, Anderson JT, Li XS, Witkowski M, DiDonato JA, Hilser JR, Hartiala JA, Haghikia A, Cajka T, et al. A terminal metabolite of niacin promotes vascular inflammation and contributes to cardiovascular disease risk. Nat Med. 2024;30(2):424–34.
Zhang Z, Liu M, Zhou C, He P, Zhang Y, Li H, Li Q, Liu C, Qin X. Evaluation of Dietary Niacin and New-Onset Hypertension among Chinese adults. JAMA Netw Open. 2021;4(1):e2031669.
Ke P, Jiang H, Dowling R, Zhong L, Ke L, Xu M, Wang C, Tian Q, He Y, Lu K, et al. Relationship between dietary niacin intake and diabetes mellitus in the National Health and Nutrition Examination Survey (NHANES) 2003–2018. Eat Weight Disord. 2022;27(7):2425–34.
Çatak J. Determination of niacin profiles in some animal and plant based foods by high performance liquid chromatography: association with healthy nutrition. J Anim Sci Technol. 2019;61(3):138–46.
Acknowledgements
The authors express gratitude to the NCHS for their exceptional efforts in creating the data for the NHANES. Additionally, they thank every participant of the NHANES for providing valuable scientific data.
Funding
This work received funding from the Youth talent science and technology project of Changzhou Health Commission (QN202109).
Author information
Authors and Affiliations
Contributions
Xingliang Feng, Yiming Chen, and Bo Zhang were involved in the conception and design. Xingliang Feng and Wei Xia conducted the analysis and interpretation of the data. Xingliang Feng and Yiming Chen contributed to the drafting of the paper. Xingliang Feng, Yiming Chen, and Bo Zhang critically revised the paper for intellectual content. All authors provided final approval of the version to be published and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Ethical approval
The NHANES protocols underwent review and approval by the National Center for Health Statistics institutional review board. All participants provided written informed consent at the time of participation. Ethical review and approval for this study were waived, as secondary analysis did not necessitate additional institutional review board approval.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Feng, X., Chen, Y., Xia, W. et al. Association between dietary niacin intake and benign prostatic hyperplasia: a population-based results from NHANES 2003–2008. J Health Popul Nutr 43, 130 (2024). https://doi.org/10.1186/s41043-024-00624-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41043-024-00624-1