Abstract
Background
Antiretroviral therapy (ART) has significantly improved the prognosis for people living with human immunodeficiency virus (PLWH). However, alterations in the gut microbiota of PLWH affect their metabolic environment and may contribute to chronic inflammation and premature aging, despite effective ART. Given that inflammation in the body have been shown to be risk factors for age-related diseases, elucidating changes in the gut microbiota and metabolic levels over time in individuals would be for understanding the dynamics in the body that lead to disease development.
Methods
The study assessed changes in blood markers, gut microbiota, and metabolic environment of PLWH on ART for four years, with participants divided into two groups based on median CD4 cell count (580/µL).
Results
The high-CD4 group was more obese than the low-CD4 group, but there was no significant difference in comorbidities, such as diabetes, hypertension, and dyslipidemia. An investigation of the alterations in gut microbiota over a 4-year period revealed a reduction in specific groups of bacteria that produce short-chain fatty acids (SCFAs) and an increase in the occurrence of Enterobacteriaceae. In addition, a decline in bacterial thiamine synthesis was predicted, and a decreased abundance of the Ruminococcus gauvreauii group, which relies on supplementation with thiamine from symbiotic bacteria, was observed. The abundance of Collinsella and Catenibacterium at baseline was positively correlated with the predicted number of thiamine-synthesizing pathway. The presence of Catenibacterium at follow-up was inversely correlated with the rate of increase in plasma levels of lipopolysaccharide-binding protein, an indicator of a leaky gut. In terms of assessing the diversity of gut microbiota, a trend towards an increase in opportunistic bacteria with different genetic distances was observed in the high-CD4 group at follow-up.
Conclusion
These observations suggested that, despite effective ART, the thiamine-deficient environment in the gut continues to reduce thiamine-requiring bacteria, such as certain SCFA-producing bacteria. As a result, secondary effects, such as proliferation of opportunistic bacteria and chronic inflammation associated with increased intestinal permeability, proceed in PLWH. Thus, certain essential nutrient deficiencies for gut metabolic environment may disrupt the symbiotic relationship between gut bacteria and increase the risk of developing inflammatory diseases.
Similar content being viewed by others
Introduction
Antiretroviral therapy (ART) has transformed human immunodeficiency virus (HIV) infection from a fatal disease to a controllable chronic condition. Despite these improvements, persistent inflammatory conditions caused by low levels of viral replication put people living with HIV (PLWH) at risk of premature aging and age-related morbidities [1]. Chronic inflammation has been linked to disease progression in PLWH, along with metabolic syndrome, osteoporosis, non-HIV-related cancers, and the development of severe comorbidities [2].
Many chronic infectious diseases, such as HIV infection and viral hepatitis, have been reported to cause alterations in the gut microbiota (dysbiosis) [3]. A decrease in short-chain fatty acid (SCFA)-producing bacteria, particularly those belonging to the genus Clostridium, observed in infections, is a characteristic feature of dysbiosis [3]. Especially, loss of SCFA-producing bacteria and decreased systemic concentrations of butyrate and propionate in the blood have been reported, and the inability to convert lactate to propionate has been linked to death and comorbidities in PLWH [4]. The gut microbiota and its byproducts impact the body’s homeostatic processes, including nutrition, metabolism, and immune system regulation, and are linked to the development of numerous disease conditions [5]. Therefore, understanding the relationship between changes in the gut microbiota during ART treatment and persistent inflammation observed in PLWH is important to prevent the risk of potential pathological progression. In many previous reports, including ours, the gut microbiota of PLWH was found to be enriched in Gammaproteobacteria, deficient in Lachnospiraceae and Ruminococcaceae, and reduced in alpha diversity, regardless of gender and sexual habits [3, 6, 7]. In other words, changes in the gut microbiota of PLWH were characterized by a decrease in the number of obligate anaerobes and an increase in the number of facultative anaerobes, suggesting a reduced intestinal barrier (resulting in leaky gut). In general, HIV disrupts the intestinal mucosal barrier, resulting in infection and depletion of CD4 + T cells, which are abundant in gut-associated lymphoid tissues [8]. This indicates that many intestinal microorganisms and their metabolites enter the blood circulation and induce immune activation and hyperinflammatory responses, contributing to chronic immune activation [9].
The gut microbiota requires growth factors, such as nucleotides, amino acids, and vitamins, for their metabolic activities. Bacteria capable of producing these growth factors are called prototrophs while those that obtained them from external sources, such as diet or cross-feeding by other bacteria, are called auxotrophs [10]. Presumably, a mutually beneficial relationship exists among gut bacteria to exchange growth factors; thiamine, also known as vitamin B1, is essential for bacterial metabolism [11] and is considered a nutrient for cross-feeding [12]. Thiamine works as a cofactor for some metabolism-related enzymes, including α-ketoglutarate dehydrogenase and pyruvate dehydrogenase [13]. Thiamine is not synthesized in humans, and is mainly obtained from dietary intake and bacterial synthesis. Research indicates that the proximal segment of the small intestine is primarily responsible for the absorption of free thiamine from dietary sources [14]. The gut microbiota generates free thiamine and thiamine pyrophosphate (TPP), both of which are absorbed by the host. Free thiamine is taken up by the colon via the thiamine transporter, similar to that in the small intestine, whereas TPP is absorbed in the colon by specific transporters [15]. Although it is unclear whether food-derived thiamine and bacteria-derived thiamine play different roles in the host, analysis using specific-pathogen-free animals would provide insights into the importance of vitamin synthesis by bacteria [16].
We had previously reported the relationship between gut bacterial profile and inflammatory status in 109 Japanese PLWH whose plasma viral load was suppressed by long-term ART treatment [6]. To emphasize the significance of understanding the intestinal environment, conducting longitudinal studies, to explore how modifications in the gut microbiota, persistent chronic inflammation, and their interplay impact metabolic changes and the gut microbiota associated with aging, would be essential. However, such studies are lacking at present. In this report, we aimed to analyze the correlation between shifts in the intestinal environment, as inferred from 4-year changes in the gut microbiota, and inflammatory cytokines in the plasma of PLWH on effective ART.
Materials and methods
Collection of blood and stool samples from study participants
The 46 participants were PLWH receiving ART treatment at the University of Tokyo Institute of Medical Science Hospital. The first (baseline) stool and blood samples were collected from study participants between 2017 and 2018 [6], and the second stool and blood samples were collected approximately four years later (follow-up). The participants were conveniently divided into two groups based on the median CD4 levels at baseline (CD4 = 580 cells/µL). Table 1 and Supplementary Table 1 provide details of the participants’ ART prescriptions and pre-existing medical conditions. All study participants had been on ART for at least 10 years, and nearly all had an HIV viral load below 50 copies/mL at baseline (45/46, 97.8%). During these 4 years, blips with detectable levels below 100 copies/mL were observed in a few patients; the viral load was otherwise under control. The main underlying diseases at baseline were diabetes (n = 3, 6.5%), hypertension (n = 9, 19.6%), and hyperlipidemia (n = 6, 13.0%) and comorbidity rates increased slightly over the four-year period (Table 1). During the study period, a small number of patients were on antibiotics, steroids, or proton pump inhibitors (PPIs) within one month prior to stool sample collection (Supplementary Table 1).
The stool samples were stored at -80 °C prior to deoxyribonucleic acid (DNA) preparation. Plasma fractions of blood samples were separated and collected in Ficoll-Paque and SepMate columns (StemCell Technologies, Vancouver, BC, Canada) and stored at -80 °C. Clinical parameters and blood samples were collected within 3 months before and after stool sample collection.
DNA extraction, amplification, and 16 S rRNA gene sequencing
Bacterial DNA was obtained from fecal samples as previously described [17]. According to the protocol outlined in the 16 S Metagenomics Sequencing Library Preparation Guide (Part #15044223 Rev. B; Illumina, San Diego, CA, USA), libraries of 16 S rRNA genes were prepared. The 16 S rRNA gene’s V3–V4 region was amplified using specific primers, including the forward primer (5 ‘-CACGACGCTCTTCCGATCTCCTACGGGNGGCWGCAG-3’) and reverse primer (5’GACGTGTGCTCTTCCGATCTGACTACHVGGGTATCTAATCC-3’), which contained Illumina adapter overhang nucleotide sequences (underlined). For PCR amplicons, adapter ligation was performed using NEBNext Multiplex Oligos for Illumina (Dual Index Primers Set 1; New England Biolabs). Sequencing was performed on an Illumina MiSeq system (Illumina) using the MiSeq Reagent Kit v3 (600-cycle) inclusive of a 15% PhiX (Illumina) spike-in.
Bacterial genome analysis
The sequences underwent a series of filtration processes, including quality assessment, noise reduction, and analysis, utilizing the Quantitative Insights into Microbial Ecology 2 (QIIME 2 version 2021.2) software, as previously documented [18]. The pipeline for the QIIME2 analysis was conducted using the following official website (https://qiime2.org/). The SILVA, a bacterial 16 S rRNA database (release 132) [19] was utilized to assign bacterial taxonomic classifications to the resulting ASVs. Specifically, the DADA2 algorithm was employed to refine paired-end reads into amplicon sequence variants (ASVs), with denoising (for example, removing miss-read sequences, low-quality reads, and primer sequences) [20]. The V3–V4 region of the 16 S rRNA gene was targeted using the naïve Bayesian classification method [21]. Statistical analysis of alpha diversity, as determined by the Shannon index, was conducted using the QIIME2 software (https://view.qiime2.org/), with a significance threshold of p-value < 0.05. To eliminate potential biases arising from variations in sequencing depth, the ASVs tables were aligned to an equal depth of 10,000 sequences per sample through alpha-rarefaction analysis. Data pre-processing was carried out using the ANCOM-II method to eliminate low-abundance or rare taxa prior to conducting differential presence ratio analysis [22]. This was followed by the Kruskal-Wallis H test for QIIME2. PERMANOVA, a permutational multivariate analysis of variance, was employed to assess the β-diversity and intricacy of bacterial communities across the samples. UniFrac distances for β-diversity, both unweighted and weighted, were presented in a distance matrix computed using QIIME2.
Statistics Analysis
The analysis of metagenomic profiles involved the use of multivariate analysis with linear models (MaAsLin)2 [23], with default parameters including a minimum prevalence of 0.1 and a maximum significance of 0.25. The MaAsLin2 analysis pipeline was conducted using the following website (https://huttenhower.sph.harvard.edu/maaslin/) as a reference. The R program (version 4.3.2) was utilized for this analysis. Phylogenetic investigation of communities by reconstruction of unobserved states (PICRUSt) 2 [24] was used to predict microbial content and functional predictions based on bacterial genes, and the Kyoto Encyclopedia of Genes and Genomes database was used to examine predicted metabolic networks of gut microbiota [25]. The pipeline for the PICRUSt2 analysis was conducted using the following website: https://huttenhower.sph.harvard.edu/picrust/ as a reference. The Wilcoxon matched-pairs signed rank test was used to test α-diversity-level and genus-level differential abundances of bacteria between baseline and follow-up, with a significance level set at p < 0.05 or False Discovery Rate corrected p < 0.05 (two-tailed). GraphPad Prism 9 software (GraphPad Software, San Diego, California, USA) was used for comparative analysis and to evaluate the relationship between bacterial compositions using the Spearman correlation test. Multiple t-tests (and nonparametric tests) were performed using Prism9 for comparative analysis of bacterial metabolic pathways and plasma cytokine/chemokine levels at baseline and follow-up.
Determination of cytokines in plasma
The Bio-Plex System from Bio-Rad Laboratories, along with the Bio-Plex Pro Human Chemokine Panel (40-Plex No. 171AK99MR2), and Bio-Plex Pro Human Inflammation Panel 1 (37-Plex No. 171AL001M), was utilized to evaluate the plasma levels of inflammatory cytokines. The assay was performed according to the manufacturer’s instructions.
Results
Collection of stool and blood samples from PLWH and their medical histories
Gut microbiota was analyzed in 2018 for 109 PLWH [6], 46 of whom had stool and blood samples collected after 4 years to analyze their gut microbiota (follow-up study). The median plasma CD4 count of study participants was 580 cells/µL. Therefore, for convenience, PLWH were classified into two groups, using the median plasma CD4 count of 580 cells/µL as the cutoff point; the group with CD4 count greater than 580 cells/µL was high-CD4 group and the other with CD4 count is or less than 580 cells/µL was low-CD4 group. Examination of the body mass index (BMI) of these two groups revealed that the high-CD4 group had a significantly higher BMI than the low-CD4 group (p = 0.045 at baseline and p = 0.011 at follow up). There were no significant differences in the prevalence of comorbidities (diabetes, hypertension, and dyslipidemia) between the two groups during research period (Table 1).
Comparative analysis of gut microbial diversity in PLWH
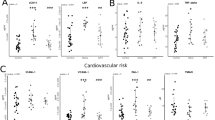
A comparative analysis of the gut microbiota at baseline and follow-up was performed for the high- and low-CD4 groups. First, a comparative analysis of three alpha diversity indices over the study period was performed to analyze the overall microbial changes; no statistically significant change was observed in the amplicon sequence variants (ASVs) or Shannon index between the baseline and follow-up for either group (Fig. 1A and B). In contrast, Faith-PD, which measures genetic distance, showed a statistically significant increase from baseline to follow-up in the high-CD4 group (Fig. 1C). However, no change was observed in the low-CD4 group. Principal coordinate analysis (PCoA) was performed to compare the overall composition (β-diversity) of the microbial community based on UniFrac distance measurements. In the PCoA-based plot, the high-CD4 group was significantly separated from baseline to follow-up in the unweighted analysis, considering only bacterial presence or absence, whereas the low-CD4 group did not (Fig. 1D). However, in the weighted UniFrac distance, which considers the observed bacterial abundance, there was no significant difference between the baseline and follow-up for both the groups (Fig. 1E).
Bacterial diversity analysis based on changes in gut microbiota at baseline and follow-up in people living with human immunodeficiency virus (PLWH). (A-C) Comparative analysis of alpha diversity of the gut microbiota in 46 PLWH at baseline and follow-up. Amplicon sequence variants (ASVs) (A), Shannon index (B), and Faith phylogenetic diversity (Faith-PD) (C). Statistical analyses were performed using the Wilcoxon matched-pairs signed rank test. (D-E) Beta diversity analysis. Principal Coordinate Analysis comparison of gut microbiota from the geographic location of PLWH at baseline and at follow-up (unweighted UniFrac distance, D) and (weighted UniFrac distance, E). B: baseline, F: follow-up, ns: not significant
Temporal changes in gut microbiota from baseline to follow-up in high- and low-CD4 groups
We compared the temporal changes in gut microbiota of the high- and low-CD4 groups over the study period. Since body weight and aging may be correlated with metabolism (21), these were considered potential confounding factors when assessing changes that may have occurred in the gut microbiota during the study period. Therefore, we performed a multivariate analysis with a linear model (MaAsLin2) adjusted for body mass index (BMI) and age (Fig. 2) and observed fluctuations in the overall gut microbiota across the five classes of bacterial groups during the follow-up period compared to that in baseline. A decrease in Collinsella and an increase in unidentified Enterobacteriaceae bacteria were observed in both the groups. A decrease in the Ruminococcus gauvreauii group was observed in the high-CD4 group during the study period, whereas a decrease in Blautia was observed in the low-CD4 group. Both bacteria were belonging to the Clostridia class that are known to produce SCFAs. Regarding the movement of other bacteria, the increase in bacteria belonging to the genera Cutibacterium, Corynebacterium1, and Staphylococcus, observed in the low-CD4 group from baseline to follow-up.
Change of gut microbiota and bacterial diversity analysis at baseline and follow-up in people living with human immunodeficiency virus (PLWH). (A) Comparative analysis of PLWH gut microbiota at baseline and follow-up at the genus level based on MaAsLin2 analysis. Participants with a CD4 count > 580 cells/µL at baseline (high-CD4 group; CD4 > 580) and those with a CD4 count 300–580 cells/µL (low-CD4 group; CD4 300–580)
Intestinal environmental changes and decrease in Collinsella: Understanding the role of thiamine synthesis
Given the common decrease in Collinsella among the changes in the gut microbiota from baseline to follow-up in the high- and low-CD4 groups, we investigated the modifications in intestinal environment that coincided with the decline in Collinsella. Bacterial functional genes were predicted using PICRUSt2 based on 16 S rRNA genes, and metabolic pathways that differed significantly between baseline and follow-up were examined. A total of 49 biological pathways, including metabolic, genetic, and environmental information processing, showed statistically significant changes between the baseline and follow-up (Table S2). Of these, six pathways were reduced at follow-up, including K14153, which was associated with thiamine synthesis. Thiamine, also known as vitamin B1, is an essential cofactor for metabolism, and B vitamins, including thiamine, have been shown to be co-utilized in bacterial communities [12, 13]. We then focused on the decrease in K14153 count at follow-up (p < 0.0001, Fig. 3A). A schematic of the thiamine synthesis pathway (hydroxymethylpyrimidine kinase, phosphomethylpyrimidine kinase and thiamine-phosphate diphosphorylaseis) corresponding to K14153 is shown in Fig. 3B. K14153 had no correlation with the changing rate of BMI (Fig. 3C), but was positively correlated with the abundance of Collinsella at baseline (Fig. 3D, left). In PLWH, a decrease in some SCFA-producing bacteria was observed from baseline to follow-up (Fig. 2). Given this information, a decrease in SCFA-producing bacteria was hypothesized to be associated with a decrease in thiamine synthesis. Therefore, we looked for other bacteria that showed a positive correlation with thiamine synthesis; Catenibacterium, a SCFA-producing bacteria, was found to show highly significant correlation with K14153 (p < 0.0001, r = 0.7252, Fig. 3D, right). Among the 46 participants in this study, a decrease in the abundance of Catenibacterium was observed from baseline to follow-up (p < 0.0001, Fig. 3E).
Predicted changes in the intestinal environment of PLWH based on bacterial changes. (A) Among the functional gene pathways in the Kyoto Encyclopedia of Genes and Genomes database, the predicted number of genes in the thiamine synthesis pathway (K14153) at baseline and at follow-up Comparative analysis. (B) Overall schematic diagram of the thiamine synthesis pathway and enzymes to which K14153 corresponds (ThiD and ThiE, blue). (C) Correlation analysis of the percentage change in BMI and K14153 from baseline to follow-up. (D) Correlation analysis between the abundance of Collinsella (left) and Catenibacterium (right) at baseline and predicted number of K14153 genes. (E) Changes in Catenibacterium abundance from baseline to follow-up. B: baseline, F: follow-up, ns: not significant
Plasma biomarkers and leaky gut in PLWH
PLWH are at an increased risk of developing age-related diseases owing to chronic inflammation and oxidative stress in their bodies [1]. To gain a deeper understanding of this increased risk, we analyzed the changes in plasma biomarkers during the study period; comparing the concentrations of 46 cytokines and chemokines between baseline and follow-up, we observed statistically significant increase in five plasma factors and decrease in 12 plasma factors. The trend of changes in inflammation levels in participants showed primarily increased plasma levels of IL-27 (p28) and IL-8 and, conversely, decreased levels of IL-6-related factors (sIL-6Ra and gp130/sIL-6b) (Table 2).
To evaluate the impact of dysbiosis on the development of leaky gut associated with inflammation in PLWH, we measured the plasma levels of sCD14 and lipopolysaccharide-binding protein (LBP). Although there was no statistically significant change in these indicators from baseline to follow-up (Fig. 4A), we found that the rate of change in sCD14 and LBP levels was negatively correlated with the ratio of Catenibacterium present at follow-up (Fig. 4B). At follow-up, plasma LBP levels positively correlated with plasma sCD163 (p = 0.03, r = 0.3649) as an indicator of macrophage activation and IFN-β (p = 0.03, r = 0.3750) (Fig. 4C). Additionally, the rate of change in LBP levels was positively correlated with plasma IL-27 (p28) (p = 0.047, r = 0.450) and IL-12 (p70) (p = 0.03, r = 0.4763) levels at the follow-up (Fig. 4D). The abundance of Cateniabcterium was inversely correlated with plasma IL-8 level at follow-up (p = 0.02, r= -0.295) (Fig. 4E).
Correlation between changes in plasma inflammatory biomarkers and gut bacteria from baseline to follow-up in PLWH. (A) plasma levels of soluble (s) CD14 and lipopolysaccharide binding protein (LBP) at baseline and follow-up Comparative analysis of (B) Correlation analysis of the rate of change in plasma LBP and sCD14 from baseline to follow-up with the amount of Catenibacterium present at baseline. (C) The relationship between plasma levels of LBP at follow-up and plasma soluble (s) CD163 and IFN-β levels was analyzed. (D) The rate of change in plasma LBP from baseline to follow-up positively correlated with plasma IL-12 (p70) and IL-27 levels at follow-up. (E) Abundance of Catenibacterium inversely correlates with Plasma IL-8 levels at follow-up
Discussion
The current study aimed to understand the changes in aging characteristics and the intestinal environment in PLWH, based on the changes in the gut microbiota of PLWH during the 4-year study period and the prediction of functional genes using their 16 S rRNA genomes. Gut microbiota was found to change during the study period, with decrease in some vitamin B1-requiring bacteria as well as in SCFA-producing bacteria. Decrease in some bacteria was correlated with a decrease in thiamine biosynthesis and was inversely correlated with an increase in intestinal permeability markers. The results of this study suggested that changes in the gut microbiota and increased intestinal permeability persist in PLWH and that vitamin deficiency is involved in the background.
In this study, a decrease in Collinsella, belonging to the Coriobacteriia class, and an increase in bacterial genera belonging to the Gammaproteobacteria class were observed as characteristics of changes in the genus-level gut microbiota throughout the study period, regardless of CD4 values. Both of these progressive changes have been reported to be related to HIV infection rather than sexual preference [7, 26,27,28], and have been indicated to correlate with inflammation [7]. This finding suggested that HIV infection-dependent dysbiosis persists despite continued ART and virological suppression. The overall trend of inflammation levels from baseline to follow-up in participants during the 4-year research period showed an increase in IL-27 and IL-8 plasma levels, indicating partial activation of intestinal inflammation. Conversely, the levels of IL-6-related factors (sIL-6Ra and gp130/sIL-6b) decreased, suggesting that the increase in inflammation levels in PLWH was not consistent. Our observations were partially consistent with a report that increased levels of the proinflammatory cytokines IL-6 and IL-8 are associated with increased levels of Proteobacteria and decreased levels of Ruminococcus lactaris and others [29]. Taken together, these findings imply that continuing changes in certain gut microbiota in PLWH may be related to persistent inflammation.
In terms of alterations in the functional characteristics of the gut microbiota from baseline to follow-up, the gut environment of PLWH demonstrated a reduction in thiamine synthesis. Our research revealed a persistent decrease in the abundance of a group of bacteria that produce SCFAs, namely Blautia and Ruminococcus gauvreauii group. A certain butyrate-producing bacteria belonging to the phylum Firmicutes do not possess a thiamine synthesis system and depend on external sources [30]. In particular, Ruminococcus spp. lacks a thiamine synthesis system [31], suggesting that the observed decrease in the Ruminococcus gauvreauii group was due to thiamine deficiency in the intestinal environment of PLWH. Given that bacteria belonging to the genus Clostridia, including SCFA-producing bacteria, are generally depleted in PLWH [6], the reduced-thiamine synthesis environment by bacteria could possibly be a contributing factor to the dysbiosis observed in these individuals. In particular, vitamin B has been suggested to be actively shared among bacteria [12]. In the present study, a positive correlation of the thiamine synthesis pathway (K14153) was observed for the SCFA-producing bacteria Collinsella and Catenibacterium, but the survival of these bacteria may be sensitive to reduced thiamine.
From an immunological perspective, thiamine is essential for the regulation of immune cell function, particularly immune metabolism [32]. Naive T and B cells, Treg cells, and M2 macrophages rely on the tricarboxylic acid (TCA) cycle, which is the most important biochemical reaction circuit related to aerobic metabolism for energy metabolism, whereas activated and inflammatory cells, such as Th1, Th2, Th17, and M1 macrophages, utilize glycolysis [33]. In intestinal immune metabolism, naive immunoglobulin (Ig) M-positive B cells of the Peyer’s patches (PP) differentiate into IgA-producing plasma cells [34]. Mice fed a vitamin B1-deficient diet have been shown to exhibit impaired maintenance of naive B cells that preferentially utilize the vitamin B1-dependent TCA cycle in the PP, resulting in reduced IgA antibody response to oral vaccines [35]. Unlike systemic immunity, the immune system of the intestinal epithelium and intestinal mucosa not only constantly monitors and eliminates microbes but also establishes a highly symbiotic relationship with the corresponding bacterial community [36]. The role of bacterial thiamine in the mechanism of microbial surveillance of intestinal epithelium by IgA is currently not clear. In the present analysis, the significant increase in Faith-PD from baseline to follow-up, observed in the high-CD4 group, indicated an increase in genetically distinct opportunistic bacteria. This finding was supported by the increase in the unweighted UniFrac distance (beta diversity) in the same comparison of the high-CD4 group. In addition, the abundance of Catenibacterium was inversely correlated with the rates of increase in LBP, a marker of intestinal permeability, and sCD14, a marker of monocyte activation. Thus, a thiamine-deficient environment in the intestinal tract may exacerbate leaky gut via changes in the intestinal microbiota, characterized by a decrease in SCFA-producing bacteria, which, in parallel, may increase opportunistic bacteria due to a decrease in mucosal barrier function. Bacteria secrete metabolites and extracellular vesicles that establish a symbiotic relationship between the host and bacteria [37]. Bacterial changes due to thiamine deficiency may contribute to the alteration of these systems. The impact of transient viral infections on the gut microbiota of PLWH is more pronounced than in healthy individuals, requiring a longer recovery time [38, 39]. However, the significance of thiamine deficiency in this context would require further investigation.
This study had several limitations. The study did not analyze gut microbiota with respect to comorbidities or PPI use. In this follow-up analysis, 35 PLWH (76%) had changed ART medications from baseline and could not be assessed based on the ART regimen. The discussion in this study was based on the genomic analysis of bacterial 16 S rRNA and not on actual measurements of the amount of thiamine and other substances present in the intestinal tract. Furthermore, long-term observations would be recommended to enhance the validity of our conclusions.
In a report of intestinal metagenomic analysis, the synthesis pathway of intestine-derived vitamin B and amino acids in PLWH was not found to be significantly restored by antiretroviral therapy [40], it may be necessary to consider ways to improve this situation. Probiotics for undernourished infants in Indonesia resulted in the recovery of SCFA-producing bacteria, mainly Collinsella and Catenibacterium, thereby increasing SCFAs in the gut [41]. Dietary supplementation with thiamine has also been shown to reduce damage to the gut tissue structure and microbial environment in goats [42]. These observations suggest that interventions, such as dietary nutrient supplementation, may be a strategy to improve the intestinal environment. Appropriate exercise may be an effective tool, as it has also been shown to promote beneficial changes in gut microbiota [43, 44]. Currently, the efficacy of ART is improving the long-term survival of PLWH, although several secondary issues due to chronic inflammation still remain a challenge [1, 2]. Strategies to mitigate thiamine insufficiency within the intestinal tract or promote the growth of SCFA-producing bacterial populations could be beneficial for addressing these concerns.
Conclusion
Our report revealed a gradual progression in dysbiosis correlated with a thiamine-deficient environment in the intestinal tract, despite effective ART in PLWH. The lack of essential nutrients in the gut metabolic environment can lead to an imbalance in the relationship between gut bacteria, potentially developing inflammatory diseases. Because a decrease in SCFA-producing bacteria is observed in many diseases, understanding this phenomenon at the molecular level may help prevent the progression of inflammatory diseases as well as premature aging.
Data availability
Data described in this study are openly available in DNA Data Bank of Japan (DDBJ) https://ddbj.nig.ac.jp/search/en; accession number: DRA012374 and DRA017294.
Abbreviations
- ART:
-
Antiretroviral therapy
- PLWH:
-
People living with human immunodeficiency virus
- SCFA:
-
Short-chain fatty acid
- ASVs:
-
Amplicon sequence variants
- PCoA:
-
Principal coordinate analysis
- BMI:
-
Body mass index
- QIIME:
-
Quantitative insights into microbial ecology
- DADA:
-
Divisive amplicon senoising algorithm
- ANCOM:
-
Analysis of compositions of microbiomes
- PERMANOVA:
-
Permutational multivariate analysis of variance
- MaAsLin:
-
Multivariate analysis with linear models
- PICRUSt:
-
Phylogenetic investigation of communities by reconstruction of unobserved states
- LBP:
-
Lipopolysaccharide-binding protein
- TCA:
-
Tricarboxylic acid
References
Rodes B, Cadinanos J, Esteban-Cantos A, Rodriguez-Centeno J, Arribas JR. Ageing with HIV: challenges and biomarkers. EBioMedicine. 2022;77:103896.
Zicari S, Sessa L, Cotugno N, Ruggiero A, Morrocchi E, Concato C, Rocca S, Zangari P, Manno EC, Palma P. Immune Activation, Inflammation, and Non-AIDS Co-Morbidities in HIV-Infected Patients under Long-Term ART. Viruses 2019, 11(3).
Mizutani T, Ishizaka A, Koga M, Tsutsumi T, Yotsuyanagi H. Role of Microbiota in viral infections and pathological progression. Viruses 2022, 14(5).
Sereti I, Verburgh ML, Gifford J, Lo A, Boyd A, Verheij E, Verhoeven A, Wit F, van der Schim MF, Giera M, et al. Impaired gut microbiota-mediated short-chain fatty acid production precedes morbidity and mortality in people with HIV. Cell Rep. 2023;42(11):113336.
Ohland CL, Jobin C. Microbial activities and intestinal homeostasis: a delicate balance between health and disease. Cell Mol Gastroenterol Hepatol. 2015;1(1):28–40.
Ishizaka A, Koga M, Mizutani T, Parbie PK, Prawisuda D, Yusa N, Sedohara A, Kikuchi T, Ikeuchi K, Adachi E, et al. Unique gut Microbiome in HIV patients on antiretroviral therapy (ART) suggests Association with chronic inflammation. Microbiol Spectr. 2021;9(1):e0070821.
Vujkovic-Cvijin I, Sortino O, Verheij E, Sklar J, Wit FW, Kootstra NA, Sellers B, Brenchley JM, Ananworanich J, Loeff MSV, et al. HIV-associated gut dysbiosis is independent of sexual practice and correlates with noncommunicable diseases. Nat Commun. 2020;11(1):2448.
Asowata OE, Singh A, Ngoepe A, Herbert N, Fardoos R, Reddy K, Zungu Y, Nene F, Mthabela N, Ramjit D et al. Irreversible depletion of intestinal CD4 + T cells is associated with T cell activation during chronic HIV infection. JCI Insight 2021, 6(22).
Sandler NG, Douek DC. Microbial translocation in HIV infection: causes, consequences and treatment opportunities. Nat Rev Microbiol. 2012;10(9):655–66.
D’Souza G, Shitut S, Preussger D, Yousif G, Waschina S, Kost C. Ecology and evolution of metabolic cross-feeding interactions in bacteria. Nat Prod Rep. 2018;35(5):455–88.
Frank RA, Leeper FJ, Luisi BF. Structure, mechanism and catalytic duality of thiamine-dependent enzymes. Cell Mol Life Sci. 2007;64(7–8):892–905.
Magnusdottir S, Ravcheev D, de Crecy-Lagard V, Thiele I. Systematic genome assessment of B-vitamin biosynthesis suggests co-operation among gut microbes. Front Genet. 2015;6:148.
Mrowicka M, Mrowicki J, Dragan G, Majsterek I. The importance of thiamine (vitamin B1) in humans. Biosci Rep 2023, 43(10).
Rindi G, Laforenza U. Thiamine intestinal transport and related issues: recent aspects. Proc Soc Exp Biol Med. 2000;224(4):246–55.
Nabokina SM, Inoue K, Subramanian VS, Valle JE, Yuasa H, Said HM. Molecular identification and functional characterization of the human colonic thiamine pyrophosphate transporter. J Biol Chem. 2014;289(7):4405–16.
Mooser C, Gomez de Aguero M, Ganal-Vonarburg SC. Standardization in host-microbiota interaction studies: challenges, gnotobiology as a tool, and perspective. Curr Opin Microbiol. 2018;44:50–60.
Mizutani T, Ishizaka A, Koga M, Ikeuchi K, Saito M, Adachi E, Yamayoshi S, Iwatsuki-Horimoto K, Yasuhara A, Kiyono H, et al. Correlation analysis between gut microbiota alterations and the Cytokine response in patients with Coronavirus Disease during hospitalization. Microbiol Spectr. 2022;10(2):e0168921.
Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA, Alexander H, Alm EJ, Arumugam M, Asnicar F, et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol. 2019;37(8):852–7.
Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glockner FO. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2013;41(Database issue):D590–596.
Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJ, Holmes SP. DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods. 2016;13(7):581–3.
Fabian Pedregosa GV, Alexandre Gramfort V, Michel B, Thirion O, Grisel M, Blondel A, Müller J, Nothman G, Louppe P, Prettenhofer R, Weiss. Vincent Dubourg, Jake Vanderplas, Alexandre Passos, David Cournapeau, Matthieu Brucher, Matthieu Perrot, Édouard Duchesnay: Scikit-learn: machine learning in Python. J Mach Learn Res. 2011;12:2825–30.
Kaul A, Mandal S, Davidov O, Peddada SD. Analysis of Microbiome Data in the Presence of excess Zeros. Front Microbiol. 2017;8:2114.
Mallick H, Rahnavard A, McIver LJ, Ma S, Zhang Y, Nguyen LH, Tickle TL, Weingart G, Ren B, Schwager EH, et al. Multivariable association discovery in population-scale meta-omics studies. PLoS Comput Biol. 2021;17(11):e1009442.
Douglas GM, Maffei VJ, Zaneveld JR, Yurgel SN, Brown JR, Taylor CM, Huttenhower C, Langille MGI. PICRUSt2 for prediction of metagenome functions. Nat Biotechnol. 2020;38(6):685–8.
Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28(1):27–30.
Armstrong AJS, Shaffer M, Nusbacher NM, Griesmer C, Fiorillo S, Schneider JM, Preston Neff C, Li SX, Fontenot AP, Campbell T, et al. An exploration of Prevotella-rich microbiomes in HIV and men who have sex with men. Microbiome. 2018;6(1):198.
Kelley CF, Kraft CS, de Man TJ, Duphare C, Lee HW, Yang J, Easley KA, Tharp GK, Mulligan MJ, Sullivan PS, et al. The rectal mucosa and condomless receptive anal intercourse in HIV-negative MSM: implications for HIV transmission and prevention. Mucosal Immunol. 2017;10(4):996–1007.
Noguera-Julian M, Rocafort M, Guillen Y, Rivera J, Casadella M, Nowak P, Hildebrand F, Zeller G, Parera M, Bellido R, et al. Gut microbiota linked to sexual preference and HIV infection. EBioMedicine. 2016;5:135–46.
Biagi E, Nylund L, Candela M, Ostan R, Bucci L, Pini E, Nikkila J, Monti D, Satokari R, Franceschi C, et al. Through ageing, and beyond: gut microbiota and inflammatory status in seniors and centenarians. PLoS ONE. 2010;5(5):e10667.
Soto-Martin EC, Warnke I, Farquharson FM, Christodoulou M, Horgan G, Derrien M, Faurie JM, Flint HJ, Duncan SH, Louis P. Vitamin Biosynthesis by Human Gut Butyrate-Producing Bacteria and Cross-Feeding in Synthetic Microbial Communities. mBio 2020, 11(4).
Park J, Hosomi K, Kawashima H, Chen YA, Mohsen A, Ohno H, Konishi K, Tanisawa K, Kifushi M, Kogawa M et al. Dietary vitamin B1 intake influences gut Microbial Community and the consequent production of short-chain fatty acids. Nutrients 2022, 14(10).
Yoshii K, Hosomi K, Sawane K, Kunisawa J. Metabolism of dietary and microbial vitamin B Family in the regulation of host immunity. Front Nutr. 2019;6:48.
Buck MD, Sowell RT, Kaech SM, Pearce EL. Metabolic instruction of immunity. Cell. 2017;169(4):570–86.
Shikina T, Hiroi T, Iwatani K, Jang MH, Fukuyama S, Tamura M, Kubo T, Ishikawa H, Kiyono H. IgA class switch occurs in the organized nasopharynx- and gut-associated lymphoid tissue, but not in the diffuse lamina propria of airways and gut. J Immunol. 2004;172(10):6259–64.
Kunisawa J, Sugiura Y, Wake T, Nagatake T, Suzuki H, Nagasawa R, Shikata S, Honda K, Hashimoto E, Suzuki Y, et al. Mode of Bioenergetic metabolism during B cell differentiation in the Intestine determines the distinct requirement for vitamin B1. Cell Rep. 2015;13(1):122–31.
Chen K, Magri G, Grasset EK, Cerutti A. Rethinking mucosal antibody responses: IgM, IgG and IgD join IgA. Nat Rev Immunol. 2020;20(7):427–41.
Liang X, Dai N, Sheng K, Lu H, Wang J, Chen L, Wang Y. Gut bacterial extracellular vesicles: important players in regulating intestinal microenvironment. Gut Microbes. 2022;14(1):2134689.
Ishizaka A, Koga M, Mizutani T, Lim LA, Adachi E, Ikeuchi K, Ueda R, Aoyagi H, Tanaka S, Kiyono H et al. Prolonged Gut Dysbiosis and Fecal Excretion of Hepatitis A Virus in Patients Infected with Human Immunodeficiency Virus. Viruses 2021, 13(10).
Ishizaka A, Koga M, Mizutani T, Yamayoshi S, Iwatsuki-Horimoto K, Adachi E, Suzuki Y, Kawaoka Y, Yotsuyanagi H. Association of gut microbiota with the pathogenesis of SARS-CoV-2 infection in people living with HIV. BMC Microbiol. 2024;24(1):6.
Park SY, Faraci G, Nanda S, Ter-Saakyan S, Love TMT, Mack WJ, Dube MP, Lee HY. Gut microbiome in people living with HIV is associated with impaired thiamine and folate syntheses. Microb Pathog. 2021;160:105209.
Kamil RZ, Murdiati A, Juffrie M, Rahayu ES. Gut microbiota modulation of Moderate Undernutrition in infants through Gummy Lactobacillus plantarum Dad-13 consumption: a Randomized double-blind controlled trial. Nutrients 2022, 14(5).
Wen K, Zhao MM, Liu L, Khogali MK, Geng TY, Wang HR, Gong DQ. Thiamine modulates intestinal morphological structure and microbiota under subacute ruminal acidosis induced by a high-concentrate diet in Saanen goats. Animal. 2021;15(10):100370.
Erlandson KM, Liu J, Johnson R, Dillon S, Jankowski CM, Kroehl M, Robertson CE, Frank DN, Tuncil Y, Higgins J, et al. An exercise intervention alters stool microbiota and metabolites among older, sedentary adults. Ther Adv Infect Dis. 2021;8:20499361211027067.
Motiani KK, Collado MC, Eskelinen JJ, Virtanen KA, Loyttyniemi E, Salminen S, Nuutila P, Kalliokoski KK, Hannukainen JC. Exercise Training modulates Gut Microbiota Profile and improves endotoxemia. Med Sci Sports Exerc. 2020;52(1):94–104.
Acknowledgements
We thank all volunteers who participated in this study; Naoko Nishiyama and Minako Iwanaga for technical assistance with the experiments; Tomoe Senkoji and Megumi Kubota for assistance in clinical data management; Tomoko Sato, Mika Kogayu, Akari Fukuda, Etsuko Nagai, and Tokiko Nagamura for assistance in clinical sample collection; Nozomi Yusa and Seiya Imoto for supporting the sequencing analysis; and Editage (www.editage.com) for English language editing.
Funding
This work was supported by the Japan Initiative for World-leading Vaccine Research and Development Centers (223fa627001h0001) (Y.S. and H.Y.), and (JP223fa627001) (A.I.) from the Japan Agency for Medical Research and Development, JSPS KAKENHI [grant numbers 21K11592 (T.Mi.), 21K07314 (M.K.), 22K20926 (A.I.) and 24K11630 (A.I.)], JST (Moonshot R&D – MILLENNIA Program) [grant number JPMJMS2025] (Y.S.), Ministry of Health, Labour, and Welfare of Japan [grant number 21HB2005] (H.Y.), and research grants from Taiju Life Social Welfare Foundation (A.I.).
Author information
Authors and Affiliations
Contributions
Conceptualization, A.I., M.K., and T.Mi.; methodology, A.I., M.K. and T.Mi.; formal analysis, A.I. and T.Mi.; investigation, A.I., T.Mi.; resources, M.K., writing—original draft preparation, T.Mi.; visualization, A.I. and T.Mi.; supervision, Y.S., T.Ma. and H.Y. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The Institutional Review Board at the Institute of Medical Science, University of Tokyo, granted approval for this study (IMSUT; approval no: Ref. No. 28-55-0330, 2019-71-0201). All procedures in this study adhered to relevant guidelines and regulations. The study followed the principles outlined in the Declaration of Helsinki, and participants provided written informed consent before enrollment for sample collection and analysis.
Consent for publication
Not applicable.
Competing interests
We have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ishizaka, A., Koga, M., Mizutani, T. et al. Thiamine deficiency underlies persistent gut dysbiosis and inflammation in people living with HIV on antiretroviral therapy. transl med commun 9, 25 (2024). https://doi.org/10.1186/s41231-024-00187-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41231-024-00187-7