Abstract
Background
Guided by the reserve capacity model, we evaluated the unique relationships between socioeconomic status (SES), reserve capacity (helplessness, self-efficacy, social support), and negative emotions on pain in patients with Rheumatoid Arthritis (RA).
Methods
The secondary analysis used baseline, cross-sectional data from 106 adults in a clinical trial comparing behavioral treatments for RA. Patients were eligible if they were ≥ 18 years old, met the ACR criteria for RA (determined by study rheumatologist), had stable disease and drug regimens for 3 months, and did not have a significant comorbid condition. Structural equation modeling evaluated the direct effects of SES, reserve capacity (helplessness- Arthritis Helplessness Index, self-efficacy -Personal Mastery Scale, social support- Social Provisions Scale) and negative emotions (stress and depressive symptoms- Perceived Stress Scale and Hamilton Depression Rating Scale) on pain (Rapid Assessment of Disease Activity in Rheumatology-RADAR & visual analog scale-VAS), and the indirect effects of SES as mediated by reserve capacity and negative emotions. The SEM model was evaluated using multiple fit criteria: χ2 goodness-of-fit statistic, the comparative fit index (CFI), the standardized root mean square residual (SRMR), and the root mean square error of approximation (RMSEA).
Results
Participants were mostly female (85%), 55.45 years old on average, self-identified as white (61%), Hispanic (16%), black (13%), and other (10%), and had RA for an average of 10.63 years. Results showed that low SES contributed to worse pain, through lower reserve capacity and higher negative emotions. Mediational analyses showed that reserve capacity and negative emotions partially mediated the effect of SES on pain. The final model explained 39% of the variance in pain.
Conclusions
The findings indicate that lower SES was related to worse clinical pain outcomes and negative emotions and reserve capacity (helplessness, social support, and self-efficacy) mediated the effect of SES on pain. A primary limitation is the small sample size; future studies should evaluate this model further in larger, longitudinal approaches. Interventions that target negative emotions in patients with low SES may facilitate better pain control with RA.
Trial registration
clinicaltrials.gov NCT00072657 01/02/2004 20/03/2009.
Similar content being viewed by others
Background
Approximately 1.3 million adults in the United States have rheumatoid arthritis (RA), which is a debilitating autoimmune, systemic and chronic condition characterized by inflammation [1,2,3]. Over time, RA affects larger joints and bodily organs and patients become at heightened risk for developing pain, in addition to comorbid medical (e.g. heart disease) and psychiatric conditions (e.g. depression). Together these symptoms further exacerbate detriments in an individual’s quality of life, disability status/ability to work, and even early death [4]. Pain, resulting from increased inflammation in joints, is widely common among RA and autoimmune populations and has been shown to significantly contribute to worse quality of life. Depressive psychological symptoms are also reported in 10–46% of individuals diagnosed with RA and is associated with increased inflammatory reactivity, significant pain, fatigue, and disability, and lower quality of life [5,6,7,8,9,10,11]. In addition to an exacerbation of disease-specific symptoms, patients with rheumatic diseases experiencing depressive symptoms and pain have also been shown to have poorer treatment adherence, disability, increased emergency room visits, and higher medical costs relative to those without depressive symptoms [11,12,13,14].
No single cause of RA has been identified or implicated to solely drive patient outcomes but rather an aggregate of biological, social, environmental, and psychological factors appears to influence the course of the condition. However, recent efforts to appreciate the disparities in patient outcomes have demonstrated the unique role of socioeconomic status (SES). SES includes a composite of individual income, the amount and type of education received, occupational prestige, and status in a hierarchical society [15, 16]. Socioeconomic determinants have been implicated in both the risk and trajectory of the condition, with those of lower SES having worse disease activity, mental and physical health, and quality of life relative to patients from higher SES [17,18,19,20]. Past research has identified a relationship between SES and clinical outcomes in inflammatory diseases, specifically RA and lupus [19, 21, 22].
While both SES and depressive symptoms have been shown to render patients with RA to poorer disease activity and overall health comes, there is a paucity of literature examining the psychosocial mechanisms of these associations, particularly in the context of internal and external psychosocial resources with pain, a primary clinical symptom of RA. The mechanisms through which SES affects health outcomes in rheumatic disease are less clear suggesting a need for greater precision in how RA is conceptualized, especially as it relates to the role of psychosocial and socioeconomic factors and identifying appropriate intervention targets. Improved understanding of the unique contributions and pathways of internal resources (self-efficacy), psychosocial factors, and parameters of disease symptomatology could inform future research of rheumatic diseases through greater precision of conceptualizations of disparate health outcomes and future interventions.
The reserve capacity model is a framework for examining how over the long-term SES may affect health disparities and overall differences in health outcomes [23]. Specifically, SES, above-and-beyond the influence of race, has been shown to have a gradient influence on health outcomes by exerting both direct and indirect effects on internal resources, which in turn, contribute to perceived stress and negative emotions. Stress and negative emotions are proposed to result in increases in patients’ inflammatory responses and consequently worse patient outcomes (e.g., greater inflammation related to increased pain symptoms or greater neurocognitive inflammation and worse psychological health). Accordingly, the reserve capacity model posits that lower SES may lead to more individually-experienced environmental and social stressors, which over time, may reduce or deplete internal (e.g., internal locus of control, optimism, hopefulness) and external (e.g., social support) psychosocial resources that may serve as protective factors in decreasing the effects of chronic stressors. While internal and external resources exist on a continuum, the lack of adequate resources to respond to the increased daily stressors diminishes the capacity of individuals to cope with stressors effectively, and ultimately contributes two-fold to health outcomes directly and indirectly. These effects may occur through increased autoimmune reactivity, negative emotions, negative behaviors, and persistent physiological arousal instability [24,25,26]. The reserve capacity framework posits that the aggregate of resource factors, not single-factors of personal resources explain the relationship between SES and health, which has not yet been assessed in pain outcomes in RA – an autoimmune disorder often resulting in disabling pain and direct susceptibility to increased stress reactivity [27].
Applying the reserve capacity model in adults diagnosed with RA, we sought to evaluate predictors of pain intensity, one of the most important patient-reported outcomes in rheumatic disease. In application, the reserve capacity model could shed light on modifiable factors that may serve as targets for clinical interventions that providers can employ with patients to possibly improve health disparities, and decrease pain and other negative health outcomes in patients with RA. Specifically, our primary objective was to evaluate a model proposing that SES would contribute to worse subjective pain intensity directly, and indirectly through the potential mediators of reserve capacity and negative emotions (see Fig. 1). We hypothesized that: (1) higher SES would be positively associated with reserve capacity; (2) higher reserve capacity would be related to lower levels of negative emotions; (3) lower levels of negative emotions would be related to lower pain intensity; (4) reserve capacity would mediate the relationship between SES and pain; and (5), negative emotions would mediate the relationship between SES and pain.
Materials and methods
Participants and procedures
This study used cross-sectional baseline data from adults with RA from the greater Southern California area who participated in a randomized clinical trial that compared behavioral treatments (tai chi chih, cognitive behavioral therapy, and a health education seminar) for RA. This study received approval from the Institutional Review Board at the study site and registered as a clinical trial (NCT00072657). Participants were recruited from 2004 to 2008 from clinic offices in the divisions of rheumatology at an academic medical center and a private hospital system and through newspaper advertisements and flyers posted in the community. After obtaining participants’ informed consent, the study rheumatologist completed a physical examination during which the diagnosis of RA was confirmed. Participants were then referred to the academic medical center for an evaluation of their clinical functioning and psychiatric status. Reports of medication use were also obtained, including analgesics/nonsteroidal anti-inflammatory drugs, biologic agents, disease-modifying anti-rheumatic drugs (DMARDs), and “other” medication (drugs for other medical conditions, including psychotropic agents). Details on the participant recruitment and evaluation process have been reported previously [28].
Eligible participants had to (a) be 18 years of age or older, (b) meet the American College of Rheumatology revised criteria for Rheumatoid Arthritis (1987 Revised Classification Criteria including ≥ 4 or more of the 7 criteria: morning stiffness, arthritis/swelling of 3 or more joints, symmetric arthritis, Arthritis of hand joints, rheumatoid modules, rheumatoid factor, radiographic changes), (c) have a stable disease-modifying drug regimen for 3 months prior to study entry, (d) have a stable disease course for 3 months, (e) be free of serious co-morbid medical conditions such as diabetes, congestive heart failure, renal failure, cancer, or fibromyalgia, that would confound interpretations of health status, (f) not be pregnant, (g) not have a serious psychiatric condition such as bipolar disorder, psychosis, or post-traumatic stress disorder, (h) not be suicidal, and (i) not have previous experience with cognitive behavioral therapy. All participants underwent a psychiatric evaluation using the Structured Clinical Interview for DSM Disorders [29], under the direction of the project psychologist and psychiatrist.
Measures
The structural model evaluated in this study (Fig. 1) was comprised of the constructs of SES, reserve capacity, negative emotions, and pain. Educational attainment and household income were used as indicators of the latent variable, socioeconomic status. Specifically, participants were asked to indicate their number of years of education and their total annual household income.
Demonstrating the mediators of the relationship between SES and health outcomes has been identified as a limitation in research. Therefore, our analyses distinguished reserve capacity mediators as the aggregate of resilience factors that have been previously identified as comprising aspects of reserve capacity [24, 27]. Reserve capacity was included as a latent variable with 3 indicators representing the Personal Mastery Scale (PMS; [30]), the Social Provisions Scale (SPS; [31]), and the Arthritis Helplessness Index (AHI; [32, 33]). The PMS is a 7-item scale that measures the extent to which an individual perceives a sense of optimism, personal control, or mastery over life outcomes. Responses are measured on a 4-point scale and total scores may range from 7 to 28, with higher scores reflecting a greater sense of personal mastery. The SPS assesses 6 functions or “provisions” that may be derived from social relationships (i.e., attachment, social integration, opportunity for nurturance, reassurance of worth, reliable alliance, and guidance). Items are rated on a 4-point scale; the total sum score may range from 24 to 96, with higher scores signifying greater perceived social support. The AHI is a 15-item questionnaire designed to measure participants’ perceptions helplessness and loss of control focusing on a patient’s external, health-related locus of control in association with their arthritis symptoms and pain. Items are rated on a 6-point scale and the sum score may range from 15 to 90. For analysis purposes, AHI items were reverse scored so that higher scores on this measure reflected less external, health-related locus of control and helplessness, and therefore higher levels of reserve capacity [23].
Negative emotion was included as a latent variable with 3 indicators representing the Perceived Stress Scale (PSS; [34]), the Hamilton Depression Rating Scale (HDRS; [35, 36]) and the Negative Affect Scale of the Positive Negative and Affect Schedule (PANAS; [37]). The PSS is a 10-item scale that assesses the degree to which participants find their lives to be unpredictable, uncontrollable, and overwhelming. Responses are measured on a 4-point scale; the sum score may range from 0 to 40, with higher scores indicating greater perceived stress. The HDRS is an observer-rated assessment of the nature and severity of mood, anxiety, neurovegetative, and cognitive symptoms associated with depression. The 17 items are rated on a 0–4 or 0–2 scale, and total scores may range from 0 to 50, with higher scores signifying the presence of more severe depressive symptoms. A trained project research assistant completed the HDRS on each research participant. The Negative Affect Scale of the PANAS contains a list of 10 mood adjectives and measures the extent to which participants experience negative affective states (e.g., anger, guilt, and nervousness). Items are rated on a 5-point scale, and higher scores represent greater subjective distress and negative affectivity.
Two indicators were used to measure the latent variable, arthritis pain: the total joint score from the Rapid Assessment of Disease Activity in Rheumatology (RADAR; [38]) and a pain visual analogue scale (VAS). The RADAR total joint score assessed joint pain/tenderness in 10 joints on the right and left sides of the body. Items are rated on a 4-point scale and total scores may range from 0 to 60, with higher scores indicating more severe joint pain. On the pain VAS, participants indicated the severity of their arthritis pain by placing a mark on a 10.0 cm horizontal line anchored by no pain (0 cm) and severe pain (10.0 cm). The pain VAS score measured the distance from the scale origin (0 cm) to point on the line marked by the participant.
Data analyses
Structural equation modeling (SEM) was used to examine the proposed model and analyses were conducted using Eqs. 6.1 [39]. The associations between medication use (i.e., analgesics/nonsteroidal anti-inflammatory drugs, biologic agents, DMARDs, and other medications) and the model indicator variables were assessed to determine their potential impact on model findings. If statistically significant, covariates of medication use related to RA disease and pain were partitioned from relevant indicators prior to analyses. The rule of a minimum of 10 cases to the number of measured variables was used in determining the adequacy of the data for testing the model [40]. Additionally, > 80% power for the regression coefficients among latent variables in the model required 85 cases. The SEM model was evaluated using multiple fit criteria: χ2 goodness-of-fit statistic, the comparative fit index (CFI), the standardized root mean square residual (SRMR), and the root mean square error of approximation (RMSEA). A statistically nonsignificant χ2 (p > .05) is suggestive of a good match between the data and the hypothesized model. A CFI value greater than 0.95 is considered evidence of a good fitting model [41]. For SRMR, a value < 0.08 is considered acceptable [42]. A RMSEA < 0.08 may also be indicative of good fit [43]. Model modifications were performed based on results from the Wald test and Lagrange multiplier (LM) test, along with theoretical considerations.
Mediation analyses examined the extent to which reserve capacity and negative emotions mediated the effect of SES on pain. First, the preconditions for mediation were assessed to confirm that SES was significantly related to pain and the hypothesized mediators (i.e., reserve capacity and negative emotions) [44]. Then, single mediator models were assessed to discern the mediating effects of reserve capacity and negative emotions separately. The 3-path mediated effect was also examined (i.e. the indirect effect from SES to pain mediated by reserve capacity and negative emotions). Statistical significance of the indirect effect, reflective of a significant decrease in the direct influence of SES on pain, was taken as evidence of mediation [44]. The significance of indirect effect estimates was calculated by EQS, based on the Sobel method [45]. Elimination of the initially significant direct effect of SES on pain indicated full mediation; partial mediation was established if the strength of this association was diminished but still significant [44]. The 3-path mediated effect was also evaluated with the joint significance test, which offers evidence of mediation provided all paths involved in the collective indirect effect are significantly non-zero [46].
Results
A total of 106 participants were included in the study. Table 1 shows demographic characteristics of the participants. The sample consisted of 90 females and 16 males, with an average age of 55.45 years and illness duration of 10.63 years. Participants came from a range of ethnicities. Whites were the most prevalent group, but participants from African-American, Hispanic, and Asian ethnicities were also represented. The sample can be characterized as middle to upper-middle class, possessing almost 16 years of education on average, and an annual income of greater than $50,000.
Preliminary analyses
Prior to testing the model, the data were screened, and results revealed a normal distribution and no multivariate outliers. Table 2 includes the intercorrelations, means, and standard deviations for all observed variables represented in the structural model. Evaluation of the relationships among the latent constructs indicated moderate to strong associations between SES and pain (r = − .563, p < .001), and between SES and the 2 posited mediators (for reserve capacity: r = .513, p < .001; for negative emotions: r = − .363, p < .001), confirming that the preconditions for mediation were present. In the assessment of covariates, use of DMARD medications was significantly associated with RADAR total joint score (r = − .334, p < .001), pain VAS, (r = − .287, p = .002), PANAS negative affect scale (r = − .232, p = .014), and HDRS (r = − .324, p < .001). The variance explained by this covariate was partitioned from the noted indicators prior to structural equation modeling analyses.
SEM results
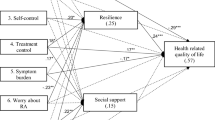
The hypothesized model (as depicted in Fig. 1) provided only a marginal fit to the observed data, CFI = 0.937; χ2(29) = 51.14, p = .007; SRMR = 0.061; RMSEA = 0.085. Post hoc modifications were performed using the LM and Wald tests in an attempt to develop a better fitting and simpler model. Based on the LM test and theoretical plausibility, the error terms for HDRS and PANAS negative affect were covaried (cov = 0.497, p < .001), which resulted in an improvement in model fit [CFI = 0.982; χ2(28) = 34.20, p = .194.; SRMR = 0.051; RMSEA = 0.046]. However, the Wald test indicated that the impact of deleting the non-significant paths from SES to negative emotions (β = 0.082, p = .616) and from reserve capacity to pain (β = 0.031, p = .905) on the χ2 of the model would be minimal. As such, in an effort to attain parsimony, these paths were removed. The fit of this revised model was similar: CFI = 0.987; χ2(30) = 34.42, p = .264; SRMR = 0.051; RMSEA = 0.037, and the model now consisted of only statistically significant paths (p < .05; Table 3). The LM test and the Wald test did not indicate any further improvement of the model through the addition or deletion of paths. Overall, the specified predictors explained 26% of the variance in reserve capacity, 50% of the variance in negative emotions, and 39% of the variance in pain. The final model with standardized path coefficients is shown in Fig. 2.
Final structural model with standardized path coefficients and factor loadings of SES, reserve capacity, and negative emotions on Rheumatoid Arthritis pain. Notes. †pathway set to 1.0. Dashes lines indicate paths dropped from model. AHI = Arthritis Helplessness Index. PMS = Personal Mastery Scale. SPS = Social Provisions Scale. HDRS = Hamilton Depression Rating Scale. PSS = Perceived Stress Scale. PANAS Negative = Negative Affect scale of the Positive and Negative Affect Schedule. RADAR Total Joint = Rapid Assessment of Disease Activity in Rheumatology total joint score. Pain VAS = Pain visual analog scale. *p < .05; **p < .01; ***p < .001
The final model of relations among SES, reserve capacity, negative emotions, and pain fit the data well. Inspection of the path coefficients showed that SES exerted a direct and negative effect on pain (β = − 0.46, p = .004). Furthermore, SES directly and positively related to reserve capacity (β = 0.51, p < .001), and greater reserve capacity predicted lower levels of negative emotions (β = − 0.71, p < .001). In turn, negative emotions had a direct and positive effect on pain intensity (β = 0.29, p = .024).
Mediation analyses
In the single mediator models, a direct relationship was specified between SES and pain, and an indirect (mediating) effect though either reserve capacity or negative emotions. The 2 single mediator models demonstrated adequate fit [CFI = 0.951; χ2(11) = 18.56, p = .069; SRMR = 0.050; RMSEA = 0.080 for reserve capacity; CFI = 1.00; χ2(10) = 8.69, p = .562; SRMR = 0.041; RMSEA < 0.001 for negative emotions]. SES was predictive of reserve capacity (β = 0.54, p = .002); however, the effect of reserve capacity on pain did not reach statistical significance (β = − 0.22, p = .210). Thus, reserve capacity did not mediate the relationship between SES and pain (βindirect = − 0.12, p = .165). While SES also had a direct effect on negative emotions (β = − 0.35, p = .025), negative emotions were predictive of pain (β = 0.31, p = .032), indicating negative emotions mediated the association between SES and pain (βindirect = − 0.11, p = .048). This finding, in combination with the attenuated but still significant direct effect of SES on pain, suggests that the association between SES and pain was partially mediated by negative emotions.
Finally, the 3-path mediated effect from SES to pain was evaluated. A test of the 3-path mediated effect of the sequence of processes depicted in Fig. 2 supported that the collective indirect effect—from SES to pain mediated by reserve capacity and negative emotions serially—was statistically explicated through the 2 mediational variables (βindirect = − 0.10, p = .029). The joint significance test also evidenced mediation because each of the 3 paths in the collective indirect effect was significant [46]. Since the direct effect of SES on pain remained significant after controlling for the 2 mediational variables, the association between SES and pain was partially mediated by reserve capacity and negative emotions.
Discussion
This study sought to evaluate the role of SES, reserve capacity, and psychological factors on pain outcomes, a highly relevant clinical symptom in patients with RA. In this sample of mostly female patients with physician-diagnosed RA, after controlling for relevant medication use, the present study demonstrated that socioeconomic status was significantly associated with worse pain intensity. Additionally, patients’ levels of reserve capacity including their reported helplessness, self-efficacy, and social support (see Fig. 2) and negative emotions significantly mediated this relationship between SES and pain intensity. Our findings are consistent with prior studies in rheumatologic clinical cohorts – lower SES was related to worse clinical outcomes including pain [47,48,49,50,51]. Novel findings here highlight that negative emotions and reserve capacity (helplessness, social support, and self-efficacy) mediated the effect of SES on pain, above and beyond the impact of patient’s RA medications, together explaining 39% of the variance in pain intensity.
Although reserve capacity was not shown to independently mediate the SES–pain relationship, results indicated that it was inversely related to negative emotions, a direct mediator of the relationship between SES and pain. Taken together, these data suggest that negative emotions result in worse pain and that high reserve capacity functions as a protective factor against negative emotions and contributes to lower pain intensity indirectly. The findings provide evidence that lower reserve capacity (higher helplessness, lower self-efficacy, and lower social support) to manage internal and external stressors results in increased negative emotions, which play a critical and unique role in explaining how lower SES results in increased pain for patients with autoimmune disorders, such as RA. In turn, higher SES can be associated with fewer and less severe environmental/social stressors and increased psychosocial reserve capacity resources (financial resources, social support, optimism, and self-esteem) to respond to these stressors, therefore leading to less inflammatory reactivity and lower negative emotions, that can then result in improved health outcomes. Prior studies evaluating a range of medical diagnoses have shown that reserve capacity resources are related to improved emotional adjustment with lower negative emotions, potentially through reduced inflammatory reactivity in the CNS, and subsequently more positive health functioning [24, 26, 52,53,54,55,56,57]. This study established the relevance of the reserve capacity framework in explaining pain outcomes in RA. It may be the case that this sample was too homogenous with limited representation of non-white individuals who also have low education and low income which may explain the lack of mediating effect of reserve capacity on SES. Yet, these findings help highlight the value of an integrated, theoretical framework that enables examination of potential underlying mechanisms to understand the pain specific inequities that result for patients with lower SES and to help identify future intervention targets.
This study has many strengths as a novel comprehensive evaluation of biological, socioeconomic, psychological, and social factors on self-reported and clinician evaluated pain in RA. The study sample was validated as patients were confirmed to have RA by physician evaluation. The study also has limitations. Although the sample was racially diverse, the majority of the patients in this sample were highly educated and above the poverty line – education and income can play an equally important role as social determinants of health [18,19,20, 48]. Yet, the mean income of participants recruited to the study was $50,262 ± 15,593, which was slightly above the median per capita income in the U.S. at that time - $47.828, making the sample similar in representation of the general U.S. public financially. Of particular note, participants were recruited from Los Angeles county, the 8th most expensive city in the world (during the recruitment period) which likely influences patients’ stress related to financial well-being. Even still, this study identified worse pain outcomes for those with lower SES. The findings can only be generalized to similar samples and future studies are needed to evaluate the mediational role of negative emotions and reserve capacity on SES and pain in larger samples with greater racial, income, and educational diversity. Lastly, the study utilized cross-sectional data which may limit the interpretability of the influence of symptoms on each other over time within the reserve capacity model. Future studies would benefit from using longitudinal designs to determine the directionality and impact of these factors on pain outcomes in RA.
Together, findings provide evidence for the unique and combined role of SES, reserve capacity factors – helplessness, self-efficacy, and social support, and negative emotions on pain outcomes in patients with RA. SES, while highly related to clinical outcomes in RA, is difficult, if not impossible, to modify. These data also support results from prior studies that show more precisely how negative emotions and reserve capacity resources together relate to rheumatologic outcomes. Pointedly, these reserve capacity resources are modifiable factors that can be the focus of behavioral interventions to facilitate better control of pain and other clinical outcomes in patients with RA [15, 58,59,60,61,62]. Comparable findings among SLE cohorts further underscore the clinical value of behavioral interventions in addressing the indirect effect lower SES may have on disease-related morbidity [48, 63]. Our findings also strengthen the path for future research that aims for greater precision in understanding how SES determinants and psychosocial factors contribute to the experience of rheumatologic conditions and patient-reported outcomes. Results support the testing of and evidence for the reserve capacity model as a model that may have utility for studying and treating rheumatologic populations more equitably. Future research is needed that can examine the effects of racial inequities, SES, negative emotions, and reserve capacity on additional parameters of disease processes in RA. More specifically, future intervention research should evaluate the potential impact of psychosocial interventions that target coping with negative emotions on relevant patient reported outcomes in RA including pain.
Data availability
De-identified data from this study are not available in a public archive. De-identified data from this study will be made available (as allowable according to institutional IRB standards) by emailing the corresponding author. Materials used to conduct the study are not publicly available.
Abbreviations
- AHI:
-
Arthritis Helplessness Index
- CFI:
-
Comparative fit index
- DMARDs:
-
Disease-modifying anti-rheumatic drugs
- HDRS:
-
Hamilton Depression Rating Scale
- LM:
-
Wald test and Lagrange multiplier test
- PANAS:
-
Negative Affect Scale of the Positive Negative and Affect Schedule
- PMS:
-
Personal Mastery Scale
- PSS:
-
Perceived Stress Scale
- RA:
-
Rheumatoid arthritis
- RADAR:
-
Rapid Assessment of Disease Activity in Rheumatology
- RMSEA:
-
Root mean square error of approximation
- SEM:
-
Structural equation modeling
- SES:
-
Socioeconomic status
- SPS:
-
Social Provisions Scale
- SRMR:
-
Standardized root mean square residual
- VAS:
-
Pain visual analogue scale
References
Helmick CG, Felson DT, Lawrence RC, Gabriel S, Hirsch R, Kwoh CK, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 2008;58(1):15–25.
Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26–35.
Almutairi KB, Nossent JC, Preen DB, Keen HI, Inderjeeth CA. The prevalence of rheumatoid arthritis: a systematic review of Population-based studies. J Rheumatol. 2021;48(5):669–76.
Odegard S, Finset A, Mowinckel P, Kvien TK, Uhlig T. Pain and psychological health status over a 10-year period in patients with recent onset rheumatoid arthritis. Ann Rheum Dis. 2007;66(9):1195–201.
Somers TJ, Keefe FJ, Pells JJ, Dixon KE, Waters SJ, Riordan PA, et al. Pain catastrophizing and pain-related fear in osteoarthritis patients: relationships to pain and disability. J Pain Symptom Manage. 2009;37(5):863–72.
Stebbings S, Herbison P, Doyle TC, Treharne GJ, Highton J. A comparison of fatigue correlates in rheumatoid arthritis and osteoarthritis: disparity in associations with disability, anxiety and sleep disturbance. Rheumatology (Oxford). 2010;49(2):361–7.
Nyklicek I, Hoogwegt F, Westgeest T. Psychological distress across twelve months in patients with rheumatoid arthritis: the role of disease activity, disability, and mindfulness. J Psychosom Res. 2015;78(2):162–7.
Feldthusen C, Grimby-Ekman A, Forsblad-d’Elia H, Jacobsson L, Mannerkorpi K. Explanatory factors and predictors of fatigue in persons with rheumatoid arthritis: a longitudinal study. J Rehabil Med. 2016;48(5):469–76.
Brandstetter S, Riedelbeck G, Steinmann M, Ehrenstein B, Loss J, Apfelbacher C. Pain, social support and depressive symptoms in patients with rheumatoid arthritis: testing the stress-buffering hypothesis. Rheumatol Int. 2017;37(6):931–6.
Kim SY, Chanyang M, Oh DJ, Choi HG. Association between depression and rheumatoid arthritis: two longitudinal follow-up studies using a national sample cohort. Rheumatology. 2020;59(8):1889–97.
Machin AR, Babatunde O, Haththotuwa R, Scott I, Blagojevic-Bucknall M, Corp N, et al. The association between anxiety and disease activity and quality of life in rheumatoid arthritis: a systematic review and meta-analysis. Clin Rheumatol. 2020;39:1471–82.
Julian LJ, Yelin E, Yazdany J, Panopalis P, Trupin L, Criswell LA, et al. Depression, medication adherence, and service utilization in systemic lupus erythematosus. Arthritis Rheum. 2009;61(2):240–6.
Manning-Bennett AT, Hopkins AM, Sorich MJ, Proudman SM, Foster DJR, Abuhelwa AY et al. The association of depression and anxiety with treatment outcomes in patients with rheumatoid arthritis – a pooled analysis of five randomised controlled trials. Therapeutic Adv Musculoskelet Disease. 2022;14.
McQuillan J, Andersen JA, Berdahl TA, Willett J. Associations of rheumatoid arthritis and depressive symptoms over time: are there differences by education, race/ethnicity, and gender? Arthrit Care Res. 2022;74(12):2050–8.
Adler NE, Ostrove JM. Socioeconomic status and health: what we know and what we don’t. Ann N Y Acad Sci. 1999;896(1):3–15.
Sule S, Petri M. Socioeconomic status in systemic lupus erythematosus. Lupus. 2006;15(11).
Margaretten M, Barton J, Julian L, Katz P, Trupin L, Tonner C, et al. Socioeconomic determinants of disability and depression in patients with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2011;63(2):240–6.
Massardo L, Pons-Estel BA, Wojdyla D, Cardiel MH, Galarza-Maldonado CM, Sacnun MP, et al. Early rheumatoid arthritis in Latin America: low socioeconomic status related to High Disease Activity at Baseline. Arthrit Care Res. 2012;64(8):1135–43.
Izadi Z, Li J, Evans M, Hammam N, Katz P, Ogdie A et al. Socioeconomic disparities in functional status in a National Sample of patients with rheumatoid arthritis. Jama Netw Open. 2021;4(8).
Salari N, Kazeminia M, Shohaimi S, Mohammadi M. Socioeconomic inequality in patients with rheumatoid arthritis: a systematic review and meta-analysis. Clin Rheumatol. 2021;40(11):4511–25.
Wolfe F, Michaud K. Out-of-pocket expenses and their burden in patients with rheumatoid arthritis. Arthritis Rheum. 2009;61(11):1563–70.
Liao KP, Alfredsson L, Karlson EW. Environmental influences on risk for rheumatoid arthritis. Curr Opin Rheumatol. 2009;21(3):279–83.
Bennett KK, Buchanan DM, Jones PG, Spertus JA. Socioeconomic status, cognitive-emotional factors, and health status following myocardial infarction: testing the Reserve Capacity Model. J Behav Med. 2015;38(1):110–21.
Gallo LC, Penedo FJ, Espinosa de los Monteros K, Arguelles W. Resiliency in the face of disadvantage: do hispanic cultural characteristics protect health outcomes? J Pers. 2009;77(6):1707–46.
Bielderman A, de Greef MHG, Krijnen WP, van der Schans CP. Relationship between socioeconomic status and quality of life in older adults: a path analysis. Qual Life Res. 2015;24(7):1697–705.
You J, Zhu Y, Liu SQ, Wang C, Wang PG, Du HF. Socioeconomic disparities in psychological health: testing the Reserve Capacity Model in a population-based sample of Chinese migrants. J Health Psychol. 2021;26(10):1538–48.
Hobfoll SE. The influence of culture, community, and the nested-self in the stress process: advancing conservation of resources theory. Appl Psychol-Int Rev. 2001;50(3):337–70.
Nicassio PM, Kay MA, Custodio MK, Irwin MR, Olmstead R, Weisman MH. An evaluation of a biopsychosocial framework for health-related quality of life and disability in rheumatoid arthritis. J Psychosom Res. 2011;71(2):79–85.
Spitzer RL, Williams JB, Gibbon M. Instruction manual for the structured clinical interview for DSM-III-R (SCID): Biometrics Research Department. New York: State Psychiatric Institute; 1989.
Pearlin LI, Schooler C. The structure of coping. J Health Soc Behav. 1978;19(1):2–21.
Cutrona CE, Russell DW. The provisions of social relationships and adaptation to stress. In: Perlman WJ D, editor. Advances in personal relationships. Greenwich, CT: JAI; 1987. pp. 37–67.
Nicassio PM, Wallston KA, Callahan LF, Herbert M, Pincus T. The measurement of helplessness in rheumatoid arthritis. The development of the arthritis helplessness index. J Rheumatol. 1985;12(3):462–7.
Callahan LF, Brooks RH, Pincus T. Further analysis of learned helplessness in rheumatoid arthritis using a Rheumatology attitudes Index. J Rhuematol. 1988;15(3):418–26.
Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–96.
Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62.
Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6(4):278–96.
Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54(6):1063–70.
Wong AL, Wong WK, Harker J, Sterz M, Bulpitt K, Park G, et al. Patient self-report tender and swollen joint counts in early rheumatoid arthritis. Western Consortium of practicing rheumatologists. J Rheumatol. 1999;26(12):2551–61.
Bentler PM. EQS structural equations program manual: Multivariate software Encino, CA; 2005.
Wolf EJ, Harrington KM, Clark SL, Miller MW. Sample size requirements for structural equation models: an evaluation of power, bias, and solution propriety. Educ Psychol Meas. 2013;73(6):913–34.
Bentler PM, Bonett DG. Significance tests and goodness of fit in the analysis of Covariance-structures. Psychol Bull. 1980;88(3):588–606.
Carmines EG, McIver JP. An introduction to the analysis of models with unobserved variables. Political Methodol. 1983;9(1):51–102.
Browne M, Cudeck R. Alternative ways of assessing model fit. In: Bollen KA, Long JS, editors. Testing structural equation models. Newbury Park, CA: Sage; 1993. pp. 136–62.
Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173–82.
MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A comparison of methods to test mediation and other intervening variable effects. Psychol Methods. 2002;7(1):83–104.
Taylor AB, MacKinnon DP, Tein JY. Tests of the three-path mediated effect. Organ Res Methods. 2008;11(2):241–69.
Lee YC, Chibnik LB, Lu B, Wasan AD, Edwards RR, Fossel AH et al. The relationship between disease activity, sleep, psychiatric distress and pain sensitivity in rheumatoid arthritis: a cross-sectional study. Arthritis Res Ther. 2009;11(5).
Yang G, Bykerk VP, Boire G, Hitchon CA, Thorne JC, Tin D, et al. Does socioeconomic status affect outcomes in early inflammatory arthritis? Data from a Canadian multisite suspected rheumatoid arthritis inception cohort. J Rhuematol. 2015;42(1):46–54.
Sumner LA, Olmstead R, Azizoddin DR, Ormseth SR, Draper TL, Ayeroff JR, et al. The contributions of socioeconomic status, perceived stress, and depression to disability in adults with systemic lupus erythematosus. Disabil Rehabil. 2020;42(9):1264–9.
Astrike-Davis EM, Cleveland RJ, Louis Bridges S Jr, Jonas BL, Callahan LF. Associations of socioeconomic status with disease progression in African americans with early rheumatoid arthritis. Arthrit Care Res. 2023;75(1):85–91.
Russell O, Lester S, Black RJ, Hill CL. Socioeconomic status and medication use in rheumatoid arthritis: a scoping review. Arthrit Care Res. 2023;75(1):92–100.
Gallo LC, Matthews KA. Understanding the association between socioeconomic status and physical health: do negative emotions play a role? Psychol Bull. 2003;129(1):10–51.
Gallo LC, Bogart LM, Vranceanu AM, Matthews KA. Socioeconomic status, resources, psychological experiences, and emotional responses: a test of the reserve capacity model. J Pers Soc Psychol. 2005;88(2):386–99.
Gallo LC, de los Monteros KE, Ferent V, Urbina J, Talavera G. Education, psychosocial resources, and metabolic syndrome variables in Latinas. Ann Behav Med. 2007;34(1):14–25.
Matthews KA, Raikkonen K, Gallo L, Kuller LH. Association between socioeconomic status and metabolic syndrome in women: testing the reserve capacity model. Health Psychol. 2008;27(5):576–83.
Schollgen I, Huxhold O, Schuz B, Tesch-Romer C. Resources for health: differential effects of optimistic self-beliefs and social support according to socioeconomic status. Health Psychol. 2011;30(3):326–35.
Boehm JK, Chen Y, Williams DR, Ryff C, Kubzansky LD. Unequally distributed psychological assets: are there social disparities in optimism, life satisfaction, and positive affect? PLoS ONE. 2015;10(2):e0118066.
Knittle K, Maes S, de Gucht V. Psychological interventions for rheumatoid arthritis: examining the role of self-regulation with a systematic review and meta-analysis of randomized controlled trials. Arthritis Care Res (Hoboken). 2010;62(10):1460–72.
Ferwerda M, van Beugen S, van Middendorp H, Spillekom-van Koulil S, Donders ART, Visser H, et al. A tailored-guided internet-based cognitive-behavioral intervention for patients with rheumatoid arthritis as an adjunct to standard rheumatological care: results of a randomized controlled trial. Pain. 2017;158(5):868–78.
Day MA, Thorn BE. The relationship of demographic and psychosocial variables to pain-related outcomes in a rural chronic pain population. PAIN®. 2010;151(2):467–74.
Thorn BE, Kuhajda MC. Group cognitive therapy for chronic pain. J Clin Psychol. 2006;62(11):1355–66.
Burns JW, Jensen MP, Thorn B, Lillis TA, Carmody J, Newman AK, et al. Cognitive therapy, mindfulness-based stress reduction, and behavior therapy for the treatment of chronic pain: randomized controlled trial. Pain. 2022;163(2):376–89.
Aberer E. Epidemiologic, socioeconomic and psychosocial aspects in lupus erythematosus. Lupus. 2010;19(9):1118–24.
Acknowledgements
The authors wish to acknowledge Mara Custodio, Kate Jackson and Sarosh J. Motivala who contributed to the diagnostic evaluation and assessment of study participants.
Funding
This work was supported by National Institutes of Health- National Institute of Arthritis and Musculoskeletal and Skin Diseases (grant number R01AR049840 NCT00072657).
Author information
Authors and Affiliations
Contributions
Conceptualization: Desiree Azizoddin, Perry Nicassio. Methodology: Desiree Azizoddin, Richard Olmstead, Michael R. Irwin, Michael Weisman, Daniel Wallace, Perry Nicassio. Project administration: Alanna E. Hirz, Mariko Ishimori, Daniel Wallace, Perry Nicassio. Data curation: Richard Olmstead. Formal analysis: Desiree Azizoddin, Richard Olmstead, Perry Nicassio. Writing- original draft preparation: Desiree Azizoddin, Richard Olmstead, Perry Nicassio. Writing- review and editing: Desiree Azizoddin, Richard Olmstead, Kris-Ann Anderson, Alanna E. Hirz, Michael R. Irwin, Shadi Gholizadeh, Michael Weisman, Mariko Ishimori, Perry Nicassio. Funding acquisition: Michael R. Irwin, Michael Weisman, Daniel Wallace, Perry Nicassio.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study was approved by the University of California Los Angeles and Cedars Sinai Medical Center Institutional Review Boards. Informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Azizoddin, D., Olmstead, R., Anderson, KA. et al. Socioeconomic status, reserve capacity, and depressive symptoms predict pain in Rheumatoid Arthritis: an examination of the reserve capacity model. BMC Rheumatol 8, 46 (2024). https://doi.org/10.1186/s41927-024-00416-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41927-024-00416-4