Abstract
Background
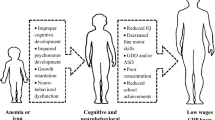
Iron deficiency anemia remains a common cause of anemia in young children. The term iron deficiency without anemia, or the so-called latent iron deficiency, has become increasingly significant as it is not only difficult to identify this condition in non-anemic children, but it also adversely affects neurocognitive development, and unfortunately, some of these effects may be irreversible and not respond to treatment. This cross-sectional study was conducted to evaluate iron status in 68 apparently healthy, non-anemic Egyptian children aged 1–6 years. They were subjected to detailed history-taking, physical examination, complete blood count, and tests for serum iron, total iron binding capacity, serum ferritin, and transferrin saturation.
Results
Low serum ferritin level and low transferrin saturation were detected in 41.2% and 47% of the children, respectively. Iron deficiency parameters were significantly affected among toddlers aged “1” to “3” years compared with preschool children, and boys were found to be more affected than girls of the same age group.
Conclusions
A normal hemoglobin level does not exclude iron deficiency, which should be screened in healthy children to prevent the possible long-term effects of iron deficiency on their cognition and mental development.
Similar content being viewed by others
Background
Iron deficiency (ID) and iron deficiency anemia (IDA) continue to be of worldwide concern. ID is the most common single nutrient deficiency among children in developing countries [1]. In developed nations, despite a marked decline in prevalence, IDA remains a common cause of anemia in young children [2]. However, the more significant than anemia itself is the more common ID without anemia that also adversely affects neurodevelopment and behavior, and some of these effects may be irreversible [3].
Iron is a trace element that is essential for numerous cellular metabolic functions [4]. The body requires iron for the synthesis of its oxygen transport proteins, in particular hemoglobin and myoglobin, and for the formation of heme enzymes and other iron-containing enzymes involved in electron transfer and oxidation–reduction [5].
Approximately 70% of the total body iron is contained in heme compounds (e.g., hemoglobin and myoglobin), 29% is stored as ferritin and hemosiderin, < 1% is incorporated into heme-containing enzymes (e.g., cytochromes, catalase, and peroxidase), and < 0.2% is found circulating in the plasma bound to transferrin [6].
There is no physiological mechanism for iron excretion, and only 1–2 mg of iron is lost each day due to sloughing of cells (i.e., from the mucosal lining of the gastrointestinal tract, skin, and renal tubules). Hence, iron loss and gain is generally in balance, with the amount lost daily being equal to the amount absorbed daily.
Hepcidin is the principal regulator of plasma iron concentrations. It acts by binding to ferroportin on cell surfaces, inducing ferroportin internalization and degradation, and thereby blocking iron efflux into the plasma from professional iron-exporting cells, including hepatocytes, duodenal enterocytes, splenic and other macrophages, and syncytiotrophoblasts [7].
The symptoms of ID are not unique to iron deficiency (i.e., not pathognomonic). The signs of ID may include brittle nails, swelling or soreness of the tongue, cracks in the sides of the mouth, an enlarged spleen, and frequent infections [8].
Structural studies from autopsies and MRI scanning have demonstrated that iron distribution in the adult brain is heterogeneous and dependent on the stage of development. The basal ganglia, substantia nigra, and deep cerebellar nuclei contain the highest concentrations of iron in the adult brain. However, in children and adolescents, the maximum iron concentrations are found in the globus pallidus, caudate nucleus, putamen, and substantia nigra, with the highest concentrations being found at birth [9].
Therefore, the timing of ID is of great significance, and it is important to consider the “critical periods” of development that absolutely require adequate iron nutrition for “normal” development. In early life, there are three peak times for the risk of developing ID, based on the balance of iron supply and demand, which are perinatal, toddlerhood, and adolescence, with the latter being particularly in females. Unfortunately, several human infant studies have demonstrated that the effects of “early” ID on biological neural functioning are potentially irreversible [10].
There are three stages of ID. The first stage is known as iron depletion, during which iron stores are low and serum ferritin concentrations decrease. IDA is the third and most severe stage of iron deficiency and is characterized by low hemoglobin and hematocrit levels [11].
There is no single reliable marker of iron status, except at the extremes of deficiency and excess [12]. A low serum ferritin level is widely considered as the best single laboratory indicator of iron depletion; the result must be interpreted with caution in any patient with an underlying inflammatory process, because ferritin is an acute phase reactant, and its level is increased in the presence of an acute or chronic inflammatory process [13].
Transferrin saturation of < 15% is indicative of an ID state, either latent ID or true ID, where a decrease in serum iron level is associated with an increase in transferrin level [14].
Screening of infants with one or more risk factors for ID would allow treatment of ID in the pre-anemic stage, thereby preventing its associated mental, motor, and behavior effects [15].
Aim of the study
The aim of our study was to evaluate iron status among apparently healthy toddlers and preschool children (aged 1–6 years) with normal hemoglobin levels to determine the proportion of children who have ID without anemia or evident clinical manifestations.
Methods
This was a cross-sectional study conducted on 68 apparently healthy children attending outpatient clinics for routine checkups during the period from January 1 to June 30, 2017. There were 21 girls and 47 boys, and they were divided into two groups as follows: toddlers aged “1” to “3” years old and preschool children aged “> 3” to “6” years old. All children were subjected to detailed history taking and complete clinical examination. The following laboratory examinations were also performed: complete blood count, including reticulocyte count, serum iron, total iron binding capacity (TIBC), serum ferritin, and transferrin saturation.
Written consent was obtained from the parents of each child involved in our study, including consent for iron therapy for children having ID.
Children were considered to be non-anemic if their Hgb levels were greater than the cutoff point established by the WHO in 2001 [16].
Results were expressed as mean ± 2 SD. Normally distributed data were analyzed using the chi-square test and analysis of variance. SPSS was used for correlation coefficient analysis and to evaluate the relationship between the variables. P < 0.05 was considered to be significant.
Results
Table 1 shows the frequency of abnormal iron indices in the examined children, where a low serum ferritin level (as the most sensitive and reliable marker of ID) was detected in 41.2% of the apparently healthy children with a normal hemoglobin level.
Table 2 shows the percentage of abnormal microscopic findings of RBCs (in blood film), where hypochromasia, microcytosis, anisocytosis, and poikilocytosis were detected in our study children.
Table 3 presents the frequency of ID in relation to age, where ID was significantly increased among children in the lower age group of 1–3 years (P < 0.05).
Table 4 shows the frequency of ID in relation to sex, where more than half of boys (51.2%) had ID versus only 19% in girls, with a significant difference (P < 0.001).
Finally, as shown in Table 5, ID was significantly higher in rural children than in urban children (P < 0.001).
Discussion
ID is the commonest form of malnutrition worldwide, and according to the WHO/UNICEF (2003) estimates, 40–50% of children aged < 5 years in developing countries are iron-deficient. ID may cause delay in the infant’s motor (activity and movement) or mental (normal thinking and processing skills) function. Management is targeted at the most vulnerable age group (preschool age) for early intervention before the condition progresses to IDA, which can result in irreversible defects in the cognitive and intellectual functions of the child [3].
In the present study, ID, diagnosed by a low serum ferritin level, was detected in 41.2% of the examined children with normal hemoglobin levels with significantly higher values among children aged “1” to “3” years. These findings add more concern to the already present major health problem of IDA in Egypt that affects > 40% of children aged < 5 years, thus increasing the risk for long-term effects of both ID and IDA on cognition and intellectual functions of these children [17]. These findings are partially consistent with the National Health and Nutrition Survey in Mexico (2006), where low iron levels were detected in 32% of children aged 12–24 months, and tissue ID was detected in 19% of children of the same age group [18]. Data from India indicated that 70–90% of children aged 6–59 months may be iron-deficient, with the prevalence being even higher in those aged < 2 years [19]. Tuohy (1994) investigated ID in preschool children in New Zealand and reported that 25% of those aged < 3 years had low iron levels. These results may be explained by the fact that children at this age go through spurts of rapid growth, which requires a large amount of iron. A balanced diet with adequate amounts of iron, which is not convenient in developing countries, is important at this age [20].
In the present study, microcytosis was observed (MCV < 75 fl) in 41.18%, hypochromasia (MCH < 24 pg) in 20.59%, and anisocytosis (RDW > 15%) in 35.29% of the examined children. The long life span of RBCs, approximately 4 months, results in several cohorts of normocytic and increasingly microcytic RBCs that coexist in the peripheral blood, leading to anisocytosis. Furthermore, ID results in poikilocytosis (variations in RBC shape), which also increases the width of RBCs [21].
In our study, ID was significantly higher among boys than girls. Our results are consistent with a study on Palestinian children aged 2–5 years, which showed that boys were more susceptible to this deficiency than girls. Sex differences in feeding practices were observed in that study, which indicated that male children were breastfed for a longer period than female children, and girls consumed more complementary foods than breast milk [24]. Increased consumption of junk foods, due to outdoor playing for a long time, in addition to poor food diversity with the consumption of large amounts of cow milk, especially in rural areas, can contribute to ID and IDA in these children.
We found a significant decrease in iron parameters in rural children compared with urban children in our study. This finding is consistent with a population-based study of Indonesian children that showed that 54% of urban children aged 6–24 months and 57% of their rural counterparts suffered from ID [19]. The high level of illiteracy, according to the general population census in 2017 reported by the Central Agency for Public Mobilization and Statistics (CAPMAS), can be definitely reflected on the lack of knowledge about healthy eating habits and the importance of dietary diversity that defiantly leads to such nutritional deficiencies [22]. Vilar-Compte et al. (2021) documented this association and confirmed that illiteracy and lower income populations tend to have less access to healthy foods and consumed less varied diets and lower amounts of vitamin C, calcium, iron, riboflavin, and zinc that directly lead to poorer nutrition outcomes [23].
Conclusions
Based on our study results, a normal hemoglobin level does not exclude ID. Hypochromasia, microcytosis, poikilocytosis, and increased RDW in the peripheral hemogram should make us focus on underlying ID even in children with normal hemoglobin levels. Toddlerhood, male gender, and residency in rural areas are the risk factors for ID. We strongly recommend conducting large multicenter studies with a larger population size in Egypt to determine the actual prevalence of this serious nutritional deficiency with an accurate determination of the underlying risk factors.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its additional file.
Abbreviations
- ID:
-
Iron deficiency
- IDA:
-
Iron deficiency anemia
- CBC:
-
Complete blood count
- TIBC:
-
Total iron binding capacity
- Hgb:
-
Hemoglobin
- EP:
-
Erythrocyte protoporphyrin
- RDW:
-
Red-cell distribution width
- MCV:
-
Mean corpuscular volume
- MCH:
-
Mean corpuscular hemoglobin
- CAPMAS:
-
Central Agency for Public Mobilization and Statistics
References
Georgieff MK (2011) Long-term brain and behavioural consequences of early iron deficiency. Nutr Rev 69:S43–S48. https://doi.org/10.1111/j.1753-4887.2011.00432.x
Sherry B, Mei Z, Yip R (2001) Continuation of the decline in prevalence of anemia in low-income infants and children in five states. Pediatrics 107:677–682. https://doi.org/10.1542/peds.107.4.677
Lozoff B, Jimenez E, Smith JB (2006) Double burden of iron deficiency in infancy and low socioeconomic status: a longitudinal analysis of cognitive test scores to age 19 years. Arch Pediatr Adolesc Med 160:1108–1113. https://doi.org/10.1001/archpedi.160.11.1108
Ram RS, Bernath PF (2003) Fourier transform emission spectroscopy of the g4Δ-a4Δ system of FeCl. J Mol Spectrosc 221:261–268. https://doi.org/10.1016/S0022-2852(03)00225-X
Hurrell RF, Reddy M, Cook JD (1999) Inhibition of non-haem iron absorption in man by polyphenolic-containing beverages. Br J Nutr 81:289–295. https://doi.org/10.1017/S0007114599000537
Zhang AS, Enns CA (2009) Molecular mechanisms of normal iron homeostasis. Hematol Am Soc Hematol Educ Program:207–214. https://doi.org/10.1182/asheducation-2009.1.207
Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J (2004) Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 306:2090–2093. https://doi.org/10.1126/science.1104742
Beard JL (2001) Iron biology in immune function, muscle metabolism and neuronal functioning. J Nutr 131:568S–579S; discussion 580S. https://doi.org/10.1093/jn/131.2.568S
Logan S, Martins S, Gilbert R (2001) Iron therapy for improving psychomotor development and cognitive function in children under the age of three with iron deficiency anaemia. Cochrane Database Syst Rev 2:CD001444. https://doi.org/10.1002/14651858.CD001444
Georgieff MK (2008) The role of iron in neurodevelopment: fetal iron deficiency and the developing hippocampus. Biochem Soc Trans 36:1267–1271. https://doi.org/10.1042/BST0361267
Allen LH (2000) Anemia and iron deficiency: effects on pregnancy outcome. Am J Clin Nutr 71(suppl):1280S–1284S. https://doi.org/10.1093/ajcn/71.5.1280s
SCAN (Scientific Advisory Committee on Nutrition) (2010) iron and health, London TSO, p 37
Arosio P, Levi S (2002) Ferritin, iron homeostasis, and oxidative damage. Free Radic Biol Med 33:457–463. https://doi.org/10.1016/s0891-5849(02)00842-0PubMed
Gross I (2004) Laboratory studies in the diagnosis of iron deficiency, latent iron deficiency and iron deficient erythropoiesis. East Maine Med Center. https://www.sabm.org/wp-content/uploads/2018/08/Anemia-in-the-Pre-Surgical-Patient.pdf.
Baker RD, Greer FR (2010) Diagnosis and prevention of iron deficiency and iron-deficiency anemia in infants and young children (0–3 years of age). Pediatrics 126:1040–1050. https://doi.org/10.1542/peds.2010-2576
WHO (2001) Iron deficiency anemia: assessment, prevention and control: a guide for programme managers. UNICEF, United Nations University, WHO, Geneva
-WHO (2011) WHO Global estimates of the prevalence of anaemia in infants and children aged 6-59 months.
Morales-Ruán Mdel C, Villalpando S, García-Guerra A, Shamah-Levy T, Robledo-Pérez R, Avila-Arcos MA, Rivera JA (2012) Iron, zinc, copper and magnesium nutritional status in Mexican children aged 1 to 11 years. Salud Publica Mex 54:125–134. https://doi.org/10.1590/S0036-36342012000200008
Sandjaja S, Budiman B, Harahap H, Ernawati F, Soekatri M, Widodo Y, Sumedi E, Rustan E, Sofia G, Syarief SN, Khouw I (2013) Food consumption and nutritional and biochemical status of 0.5–12-year-old Indonesian children: the SEANUTS study. Br J Nutr 110(suppl. 3):S11–S20. https://doi.org/10.1017/S0007114513002109
Crampton P, Farrell A, Tuohy P (1994) Iron deficiency anaemia in infants. N Z Med J 107:60–61
Handin RJ, Lux SE, Stossel TP (2003) Blood: principles and practice of hematology, 2nd edn. Lippincott Williams & Wilkins, Philadelphia, p 2304
Central Agency for Public Mobilization and Statistics (CAPMAS) announces the result of Egypt Census 2017 (30 September, 2017).
Vilar-Compte M, Burrola-Méndez S, Lozano-Marrufo A, Ferré-Eguiluz I, Flores D, Gaitán-Rossi P, Teruel G, Pérez-Escamilla R (2021) Urban poverty and nutrition challenges associated with accessibility to a healthy diet: a global systematic literature review. Int J Equity Health 20:40. https://doi.org/10.1186/s12939-020-01330-0
Acknowledgements
None.
Funding
The authors have no financial relationships relevant to this article to disclose.
Author information
Authors and Affiliations
Contributions
All authors read and approved the final manuscript. OE, KA, and FA designed the study, did the literature search, interpreted the data, and wrote the manuscript. MS shared in the study design, did all statistical analysis, and shared in the literature search and writing. MA did all the laboratory study for our studied children.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the ethics committee of the Faculty of Medicine, Assiut University (IRB no: 17101475). Written informed consents were taken from parents with explanation of benefits of the study, risks expected, and suggested treatment for each case.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Asheer, O.M., Naeem, M.S., Abdel-Hafez, F.A. et al. Iron deficiency in preschool non-anemic Egyptian children. Egypt Pediatric Association Gaz 69, 32 (2021). https://doi.org/10.1186/s43054-021-00081-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43054-021-00081-z