Abstract
Background
In the pathophysiology of the retina, the epithelial–mesenchymal transition and oxidative stress play key roles. To find a relationship between oxidative stress and diabetic retinopathy or proliferative retinopathy, we used an in-silico approach. We also used an in vitro investigation to examine the synergistic effect of transforming growth factors-β2 (TGFβ2) and hydrogen peroxide (H2O2) on the epithelial–mesenchymal transition (EMT) of ARPE-19 cells, analyzing cell survival, cell migration by wound healing, and expression epithelial–mesenchymal transition markers at the protein and mRNA levels.
Results
For 24 h, human retinal pigment epithelial cells (ARPE-19) were cultured in standard conditions and subjected to various concentrations of TGFβ2, H2O2, and their combinations. According to a bioinformatics study, signaling pathways including hypoxia-inducible factor (HIF-1), forkhead box O (FoxO), phosphoinositide-3-kinase (PI3K-Akt), and transforming growth factors-β (TGFβ-signaling), both growth factors and oxidative stress-associated proteins play a significant role in the development of retinopathies. In vitro studies showed that oxidative stress induced by H2O2 and TGFβ2 enhances the epithelial–mesenchymal transition of ARPE-19 cells.
Conclusions
Hydrogen peroxide enhances the TGFβ2-mediated epithelial–mesenchymal transition of human retinal pigment epithelial cells, or at least in cell line ARPE-19 cells.
Graphical abstract

Similar content being viewed by others
1 Background
The retinal pigment epithelium (RPE) cells are a single layer of highly polarized cells located between the choroid and photoreceptors. Each adult human eye has around 3.5 106 cells of the retinal pigment epithelium. Retinal pigment epithelium cells are traditionally assumed to undergo terminal differentiation throughout life [1, 2]. Retinal pigment epithelium cells are essential for maintaining healthy retinal physiology and the different signaling cascades required for optimal vision. They also have a significant impact on the movement of nutrients from the choroid to the inner retinal membranes and the absorption of extra dispersed light to prevent photooxidation [3]. The blood-retinal barrier (BRB), a monolayered cuboidal epithelium held together by cell–cell junctional structures such as tight junctions and adherens junctions, is a function of the retinal pigment epithelium cells in addition to their role in metabolizing retinoid molecules in the visual cycle [4, 5]. Along with maintaining the milieu, the RPE also secretes cytokines that, in some circumstances, might lead to pathological alterations such as macrophage recruitment [6].
In the human body, mesenchymal cells and epithelial cells differ in their characteristics and roles. Expression of junctional complexes and apical-basal polarization are two key characteristics of terminally differentiated epithelial cells. A basal membrane surrounds epithelial cells, separating them from other tissue constituents. Mesenchymal cells, as opposed to epithelial cells, are non-polarized cells with invasive and migrating activity [2, 7, 8]. Many biological processes, including embryogenesis, tissue fibrosis, and cancer metastasis, include the epithelial-to-mesenchymal transition. Loss of cell polarity and adhesion during the transition from epithelial to mesenchymal tissue causes morphological alterations in the cells as well as increased motility and size [9] [10]. Type I EMT occurs in the early phases of embryogenesis, Type II EMT is linked to tissue regeneration and organ fibrosis, and Type III EMT occurs in cancer cells and promotes invasion and metastasis [2, 3, 8]. As mentioned previously, the epithelial–mesenchymal transition is brought on by abnormal stimuli and a modified microenvironment, and it commonly results in tissue or organ fibrosis [11,12,13,14,15]. A growing body of research demonstrates that various degenerative retinal illnesses cause the retinal pigment epithelial cells to become less differentiated, undergo EMT, and then migrate more easily [16, 17] [3]. Epithelial–mesenchymal transition affects proliferative retinopathy, proliferative diabetic retinopathy, and other degenerative illnesses of the retina [15, 18]. Clinical evidence suggests that macular degenerations and proliferative vitreoretinopathy (PVR) cause retinal pigment epithelial cells to undergo EMT [2, 3, 17]. Loss of vision in age-related macular degeneration (AMD) may come from the breakdown of the BRB, choroidal neovascularization (CNV), and associated exudative fluids invading the subretinal region; aberrant cytokine release may also accelerate disease activity [6].
Oxidative stress is defined as an imbalance between levels of reactive oxygen species and antioxidants [19]. An increase in intracellular reactive oxygen species production can result in oxidative stress [9]. Reactive oxygen species are produced by regular cellular metabolism (mitochondrial electron transport chain) as well as in response to cytokines and environmental stimuli (radiation). Organ fibrosis may be significantly influenced by moderate chronic oxidative stress [20]. Damage caused over time to vital macromolecules by oxidative stress is one of the key factors in age-related diseases. The retina is particularly prone to oxidative stress-induced injury because of its elevated oxygen tension, ongoing exposure to light, and high concentration of polyunsaturated fatty acids (PUFA) [21, 22]. Numerous eye conditions, including proliferative vitreoretinopathy and diabetic retinopathy, as well as macular degenerative retinal diseases, are caused by oxidative stress [6, 23, 24]. One of the reactive oxygen species is hydrogen peroxide, and evidence has revealed that it is more prevalent in the aqueous humor of human eyes with proliferative retinopathies like proliferative diabetic retinopathy and proliferative vitreoretinopathy [25]. Superoxide dismutase, catalase, and glutathione peroxidase have all been found in retinal tissues like retinal pigment epithelium cells and photoreceptors [19, 26, 27].
Transforming growth factor beta (TGF-β), a subclass of cytokines controls many cellular processes, including cell division, proliferation, and differentiation [28,29,30]. Both oxidative stress/Reactive Oxygen Species (ROS) and transforming growth factor-beta (TGF-β) play crucial roles in health and disease [31]. ROS production can be boosted by TGF-β in both transformed and non-transformed cells. Furthermore, numerous studies have demonstrated that TGF-β can stimulate the production of ROS in various cellular compartments [31, 32]. There is growing evidence to support the idea that ROS induction is the mechanism by which TGFβ-induced EMT is regulated [2, 33]. Particularly during pathological conditions linked to the fibrosis of ocular structures, including retina pigment epithelial cells, TGFβ2 concentration was found to increase [15, 34,35,36]. Transforming growth factors beta (TGFβ) family proteins are essential for the epithelial–mesenchymal transition and associated organ fibrosis, such as proliferative vitreoretinopathy [37, 38].
It is still unknown what causes the epithelial to mesenchymal transition across this continuum. RPE dysfunction is described in this context as the transition from fully differentiated epithelial cells to mesenchymal cells (including the intermediate states). Aging, the breakdown of tight junctions, the buildup of misfolded protein, and inflammation are possible mechanisms underlying RPE dysfunction [2, 3, 16, 17]. Thus, the onset of retinopathies is associated with both the epithelial–mesenchymal transition and oxidative stress. Multiple studies have demonstrated that β2 exposure causes oxidative stress [15, 31, 39].
We hypothesized that elevated ROS and TGFβ together trigger retinal pigment epithelium changes from epithelial to mesenchymal states. This theory was tested in the current work, which looked at the molecular processes behind the epithelial–mesenchymal transitions of the retinal pigment epithelium caused by hydrogen peroxide and TGFβ-2. According to this work, TGFβ2-mediated processes connect oxidative stress to epithelial–mesenchymal transitions of the retinal pigment epithelium for the first time. The goal of the current work is to identify shared pathways between epithelial–mesenchymal transition and oxidative stress in retinal pigment epithelium cells using in-silico (bioinformatics) techniques. Additionally, utilizing in vitro studies on the RPE cell line ARPE-19, we investigated how TGFβ2 and H2O2-associated factors affected cell migration and the expression of epithelial–mesenchymal transition markers at the cellular, mRNA, and protein levels.
2 Methods
2.1 Materials
ARPE-19, a human retinal pigment epithelial cell line was purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). Dulbecco’s modified eagle medium (DMEM/F12) (Thermifisher, MA), fetal bovine serum (FBS) (ThermoFisher), hydrogen peroxide (Merck), TGFβ2 (Peprotech, USA) were purchased from respective sources. The primary antibodies were purchased from Cell Signaling Technology (CST, Beverly, MA, USA). The nitrocellulose membranes were purchased from Millipore, USA. The bicinchoninic acid assay (BCA) (Sigma) kit and the chemiluminescence kit were purchased from ThermoFisher (Rockford, IL, USA).
2.2 Identification of oxidative stress and diabetic retinopathy-associated potential genes
The GeneCards database (https://www.genecards.org) was used to identify genes associated with oxidative stress based on relevance scores > 10, a cutoff suggesting a considerably high link with oxidative stress. GeneCards is a freely available database that offers in-depth details on predicted and annotated human genes [40]. Proliferative vitreoretinopathy and potential diabetic retinopathy genes (C0011884) were discovered using the DisGeNET database (https://www.disgenet.org/). It incorporates knowledge of genetic variations and genes linked to human disease [41]. In addition, the PharmMapper program (http://www.lilab-ecust.cn/pharmmapper/) was used to find proteins linked to glutathione.
2.3 Construction and analysis of protein–protein interaction (PPI) networks
At the highest confidence level, protein–protein interaction networks of oxidative stress-associated potential genes, proliferative vitreoretinopathy, and diabetic retinopathy were created using the string database version 11.5 [42].In order to investigate the relationship between oxidative stress-associated proteins and proliferative vitreoretinopathy as well as diabetic retinopathy, integrated networks were created using the “merge” tool (intersection option was selected) of Cytoscape software version 3.8.2 [43]. Using Database Annotation, Visualization, and Integrated Discovery (DAVID) version 6.8 (https://david.ncifcrf.gov/), the proteins of a combined oxidative stress-diabetic retinopathy and combined oxidative stress-proliferative vitreoretinopathy protein–protein interaction network were used to perform the KEGG pathway enrichment analysis to explore their functions in the human body. The objective of this open-source online application is to offer functional annotation for lengthy lists of target genes and proteins [44].
2.4 Cell culture of ARPE-19 cells
The ARPE-19 human retinal pigment epithelial cell line was grown in DMEM/F12 with 10% FBS and antibiotic penicillin (ThermoFisher) at 37 °C, 5% CO2 in a 60-mm cell culture dish (Corning, Tewksbury, MA). Trypsin-ethylenediamine tetraacetic acid (trypsin–EDTA; ThermoFisher) was used to detach cells. Different concentrations of either only-H2O2, only-TGFβ2, or H2O2 and TGFβ2 together were administered to the cells. Untreated cells were used as a control for the experiment.
2.5 Cytotoxicity assay
By using the MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide) test, the vitality of ARPE-19 cells was evaluated [45]. In 96-well plates, 1 × 104 cells of the cells were plated in each well. For 12 h, cells were starved in a serum-free medium. Following that, cells were incubated for 24 h with various doses of only-H2O2, only-TGFβ2, and their combinations. TGFβ2 (1–10 ng/ml) and H2O2 (10–1000 µM) final concentrations were produced in a serum-free medium. Following treatment, the cells undergo a 4-h of incubation with 0.5 mg/ml MTT reagent. The formazans were then dissolved in 100 μl of dimethyl sulfoxide (DMSO) at 37 °C, and the absorbance was measured with a microplate reader (Bio-Tek Epoch microplate spectrophotometer, Vermont, USA) at 570 nm. Results were expressed as a percentage of cells that survived, with the control considered as 100%. The cell viability was calculated as described previously by Shukal and coworkers [46].
2.6 Cell migration assay
The ARPE-19 cells were grown in 12 well plates (Corning, NY) at a density of 3 X 105 cells per well until they reached 95 percent confluency. For 12 h before the treatment, cells were serum starved. With the help of a 200 µl tip, a wound was made in the monolayer of cells. To prevent any interruptions to data collection, the detached cells were eliminated using a phosphate-buffered saline (PBS) wash (ThermoFisher). For 24 h, cells were exposed to treatments of only-H2O2, only-TGFβ2 alone, and their combinations. Cells were observed under an inverted microscope (Zeiss, Germany) and 10 photographs were taken from each well using a 5X objective lens at 0 h and after completion of treatment duration of 24 h. Images were analyzed for wound length using ImageJ software (V1.45 NIH, Bethesda, MD, USA) according to the formula described previously by Shukal and colleagues [46].
2.7 RT-PCR, cDNA synthesis, and mRNA expression
As previously noted, the culture of ARPE-19 was carried out up to 75–80% confluency. Cells were treated with just H2O2, solely TGF2, and their mixtures for 24 h after serum starvation. After the course of treatment was complete, RNA was isolated using Trizol reagent (Life Technologies, Waltham, MA, USA) in accordance with the manufacturer's instructions, and 1 g of the isolated RNA was used for cDNA synthesis using Versa cDNA synthesis kit (ThermoFisher, USA) following the manufacturer's instructions. SYBR green master mix (Roche, USA) was used to quantify the gene's expression under the manufacturer's instructions on a LightCycler II, Roche, USA. Table 1 describes the list of primers. The results of mRNA expression were calculated by 2−ΔΔCt value by using GAPDH as a housekeeping gene [47].
2.8 Immunofluorescence observation
ARPE-19 cells were plated on coverslips in twelve well culture plates at 75–80% confluence. After treatment duration, coverslips were briefly washed using PBS (ThermoFisher). Cells were fixed in 4% paraformaldehyde (PFA) prepared in phosphate buffer saline (PBS) for 5–6 min. Cells were permeabilized by TritonX-100 in PBS (0.5%) for 30 min at 37 °C. Cells were blocked with 1% bovine serum albumin (BSA, ThermoFisher) in PBS. Cells were exposed to the primary antibody (dilution of 1:100) overnight at 4 °C (Table 2). The blank samples were also processed similarly except for the use of a primary antibody. Then cells were washed with Tween-20 in PBS (0.1%) three times. Cells were exposed to a secondary anti-mouse antibody tagged with Alexa Fluor488 or an anti-rabbit antibody tagged with Alexa Fluor 546 (Dilution 1:100) for 45 min at 37 °C. Then coverslips were washed five times with PBST and counterstained with 4′,6-diamidino-2-phenylindole (DAPI) (Sigma), mounted in 1,4-diazabicyclo [2.2.2] octane (DABCO), and observed under the microscope (Zeiss, Germany). Photographs were taken with a charge-coupled device (CCD) camera (Cohu).
2.9 Western blotting
In six-well plates, ARPE-19 cells were plated and allowed to reach 75–80% confluence before being used in western blotting assays. Following the completion of the treatment duration, the cells were rinsed in cold PBS, and the plate was maintained frozen. Cells were harvested using the 1X sample buffer. After a quick sonication, the cell lysate was centrifuged at 12,000 g for 10 min at 4 °C. The amount of total protein was measured using the BCA protein assay kit. In Bis–Tris gradient gels (ThermoFisher), the same quantity of protein was loaded into each well. The gels were then subjected to further electrophoresis (100 V) until the markers were well separated. Following that, proteins were transferred using a transfer buffer to the nitrocellulose membrane. Blotting was followed by blocking (5% BSA) and a primary antibody incubation at 4 °C for an overnight period while rotation. After being exposed to an anti-rabbit or anti-mouse antibody that had been horseradish peroxidase (HRP)-tagged for an hour at 37 °C, the membrane was then washed with tris-buffered saline with 0.1% Tween® 20 detergent (TBST), and bands were seen using chemiluminescence ECL substrate (BioRad). The images were captured using a gel doc system, and band intensities were calculated using ImageJ software (version 1.45, NIH, Bethesda, Maryland, USA). Results expressed as band intensities divided by GAPDH, where GAPDH served as the housekeeping gene.
2.10 Total glutathione estimation
The total glutathione was measured by the method of Rahman et al., 2007 based on the principle that 5,5′-dithiobis (2-nitrobenzoic acid) (DTNB, Ellman’s reagent) reacts with all thiol-containing substances [48]. After completion of the treatment duration, cells were processed immediately after taking from the cell culture and further, all the procedure was carried out over the ice bath. Cells were taken in a known amount of phosphate buffer (0.1 M, pH 7.4) and sonicated for two rounds of 5 s, centrifuged at 12,000 g, 10 min at 4 °C. The resultant supernatant was pipette carefully used further for the enzymatic and estimation. The total 200 µl reaction volume which consists of 120 µl of DTNB: GR (60:60 v/v mixture), 60 µl β-NADPH, and 20 µl sample aliquots then immediately readings were taken at 412 nm for every 30 s in a total of 120 s. The system devoid of samples was considered blank. The total GSH is expressed as micromole per milligram of protein. The change in absorbance was measured at 412 nm. The concentration of glutathione was calculated by the linear regression method based on the glutathione standard. The concentration of total glutathione is expressed as µM/mg protein. The BCA protein assay kit was used for total protein quantification.
2.11 Statistical analysis
Graphs were made using GraphPad Prism software (version 5.01). Data are presented as the means ± SEM. The analysis of variance (ANOVA) was utilized to find the difference between the groups. Statistical significance was set at p < 0.05. All experiments were repeated independently at least seven times.
3 Results
3.1 Network pharmacology and gene ontology analysis
A total of 428 oxidative stress, 646 diabetic retinopathy, 180 proliferative vitreoretinopathy-associated genes, and 300 glutathione-associated proteins were identified from GeneCards and DisGeNET databases. These identified potential genes were used to construct their protein–protein interaction networks using the STRING database. The resultant protein–protein interaction networks of oxidative stress, diabetic retinopathy, and proliferative vitreoretinopathy showed 424 nodes/2122 edges, 123 nodes/534 edges, and 170 nodes/244 edges respectively by eliminating unconnected nodes. Additionally, the combined oxidative stress-diabetic retinopathy, oxidative stress-proliferative vitreoretinopathy, and oxidative stress-glutathione protein–protein interaction networks were developed to explore the connection between them which showed that the protein–protein interaction networks network model possessed 123 nodes/534 edges, 33 nodes/77 edges, and 38 nodes/76 edges respectively (Fig. 1A–C).
Combined oxidative stress-diabetic retinopathy protein–protein interactions network (1-A). Combined oxidative stress-proliferative vitreoretinopathy protein–protein interactions network (1-B). Combined diabetic retinopathy-glutathione protein–protein interactions network (1-C). The constructed combined oxidative stress-diabetic retinopathy with 123 nodes and 534 edges. Nodes represent a protein and edges represent the connection between two proteins
To illustrate the effect of oxidative stress-associated proteins in diabetic retinopathy as well as proliferative vitreoretinopathy, the proteins of combined oxidative stress and diabetic retinopathy, protein–protein interaction networks were submitted to the DAVID web server to execute the KEGG pathway enrichment analysis. The results of the KEGG pathway enrichment analysis demonstrated that oxidative stress-associated proteins play a critical role in the development of diabetic retinopathy as well as proliferative vitreoretinopathy through regulating signaling pathways such as HIF-1, tumor necrosis factor (TNF), FoxO, PI3K-Akt, mitogen-activated protein kinase (MAPK), Toll-like receptor, T-cell receptor, vascular endothelial growth factor (VEGF), Estrogen, nuclear factor kappa B (NF-kappa B), Janus kinase/signal transducer and activator of transcription signaling pathway (Jak-STAT) and TGF-β (Table 3). In addition, in silico analysis suggested the involvement of potential target proteins of glutathione are associated with oxidative stress by targeting HIF-1, TNF, FoxO, PI3K-Akt, MAPK, Toll-like receptor, T-cell receptor, VEGF, Estrogen, and Jak-STAT signaling pathways.
3.2 Effect of H2O2 and TGFβ2 on the Survival of ARPE-19 cells
ARPE-19 cells were exposed to different concentrations of H2O2 (Fig. 2) at 10, 20, and 30 μM H2O2, there was no significant cell death (at 10 μM p < 0.001, at 20 μM p < 0.001, and 30 μM, p < 0.05). At 40 μM H2O2, there was very little effect but after 40 μM, there was a reduction in the survival of cells as H2O2 concentration of increased (p < 0.001 for all concentrations above 40 μM). TGFβ2 treatment on ARPE-19 cells for 24 h showed a non-significant effect on cell survival (from 1 to 10 ng/ml TGFβ2). The simultaneous treatment of TGFβ2 and H2O2 was dependent on the concentration of H2O2. Treatment of H2O2 at 10, 25 μM, and 100 μM along with 10 ng/ml TGFβ2 showed less cell death compared to that of similar concentration only-H2O2 treated cells. However, a higher concentration of H2O2 (400 μM) along with 10 ng/ml TGFβ2 showed significantly reduced cell survival compared to that of a similar concentration of only-H2O2 treated cells. Based on the above results, we have selected 10 ng/ml TGFβ2 and 10, 25, 100, and 400 μM H2O2 for further analysis.
Effects of H2O2 and TGFβ2 on the survival of ARPE-19 cells. Exposure of H2O2 after 40 μM, the OS-dependent reduction in cell survival observed compared to control (*** = p < 0.001). The Exposure of only-TGFβ2 (1–10 ng/ml) showed the non-significant change in cell survival compared to the untreated control. There was no cell death observed when the treatment of TGFβ2 (10 ng/ml) when it given simultaneously with H2O2 (10, 25 μM and 100 μM) compared to that of similar concentrations of only-H2O2 treated cells. However, at higher concentrations of H2O2 (400 μM) reduced the survival observed compared 10 ng/ml TGFβ2 treated cells. n = 7 for each group. Data represented by mean ± SEM as a percentage to that of untreated cells. All TGFβ2 and H2O2 concentrations tested are compared with untreated cells and shown with ns, non-significant, * = p < 0.05, ** = p < 0.01, and *** = p < 0.001. The combination of TGFβ2 and H2O2 are compared with similar concentration of H2O2 and represented as $ = p < 0.05, $$ = p < 0.01, and $$$ = p < 0.001 for 10 ng/ml TGFβ2
3.3 Effect of H2O2 and TGFβ2 on the migration of ARPE-19 cells
Exposure of only-TGFβ2 led to elongated cells compared to that of untreated cells while only-H2O2 does not show any cell elongation. The morphology of cells in H2O2 along with TGFβ2 treated cells showed elongation (Fig. 3). The wound-healing assay was performed to assess the effect of H2O2 on the migration of ARPE-19 cells. The ARPE-19 cells were treated with either TGFβ2 (10 ng/ml) or H2O2 (10, 25,100,400 μM) and their combinations for 24 h. Figure 2 shows the representative images of the ARPE-19 wound healing response. TGFβ2 (10 ng/ml) along with H2O2-exposure increased the wound healing response of cells compared to the untreated groups (Fig. 2). H2O2 at 10, 25, and 100 μM showed higher wound closure compared to that of the untreated group. However, 400 μM H2O2 led to reduced wound closure than untreated cells. Based on the above results, we have selected 25 μM and 100 μM H2O2 for further evaluation.
Effects of H2O2 and TGFβ2 on the wound healing of ARPE-19 cells. Exposure of TGFβ2 led to elongated cells and increased wound closure compared to that of untreated control cells. High concentration of H2O2 at 400 μM reduced the migration of cells and hence reduced the wound closure. The simultaneous treatment of H2O2 (10 μM, 25 μM, 100 μM) along with TGFβ2(10 ng/ml), the increased wound closure was observed compared to untreated and only-H2O2 treated cells and showed elongated cells. However, at 400 μM H2O2 along with 10 ng/ml TGFβ2, the wound closure was reduced than that of only H2O2. n = 7 for each group. Data represented by mean ± SEM as a percentage to that of untreated cells. All TGFβ2 and H2O2 concentrations tested are compared with untreated control cells and shown with ** = p < 0.01, and *** = p < 0.001. The combination of TGFβ2 and H2O2 was compared with a similar concentration of only H2O2 and represented as # = p < 0.05, and ## = p < 0.01
3.4 Effect of H2O2 and TGFβ2 on the expression of epithelial–mesenchymal transition markers
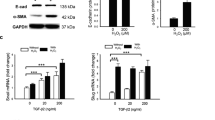
The effects of H2O2 and TGFβ2 is evaluated at mRNA, cellular, and protein levels. The results of mRNA expression are shown in Fig. 4. We have used zonula occludens-1 (ZO1), E-cadherin, β-catenin, and Pax6 as epithelial/RPE markers. H2O2 exposure at 25 μM did not alter the expression of ZO1, E-cadherin, and β-catenin (p < 0.05) and increased expressions of Pax6 (p < 0.01) compared to untreated control groups. H2O2 exposure at 100 μM reduced the expression of ZO1 (p < 0.05), E-cadherin (p < 0.05), and β-catenin (p < 0.01). TGFβ2 at 10 ng/ml reduced the expression of all epithelial/RPE markers compared to that of untreated cells. The simultaneous treatment of TGF β2 and H2O2 furthermore significantly reduced the expression of all epithelial/RPE markers compared to that of respective concentrations of only-H2O2. We have used fibronectin, alpha-smooth muscle actin (α-SMA), S100A4, and matrix metallopeptidase 9 (MMP9) as a marker of epithelial–mesenchymal transition. The simultaneous treatment of TGFβ2 (10 ng/ml) and H2O2 (25 μM and 100 μM) elevated the expression of all epithelial–mesenchymal transition markers compared to that of respective concentrations of only-H2O2.
Effects of H2O2 and TGFβ2 on the expression of various epithelial and epithelial–mesenchymal transition markers at mRNA level in the ARPE-19 cells. The mRNA expression of epithelial markers ZO1, E-cadherin and β-catenin reduced with the simultaneous exposure H2O2 and TGFβ2 compared to untreated control cells. Similarly, the Pax6 mRNA expression also reduced compared to untreated cells. The combined effect of H2O2 with TGFβ2 increased expression of mesenchymal-markers (fibronectin, N-cadherin, α-SMA, S100A4, MMP9) compared to that of only-H2O2. n = 7 for each group. Data are represented as a percentage of control as mean ± SEM. H2O2 alone and TGFβ2 alone exposures were compared with the untreated cells and shown with ns = non-significant, * = p < 0.05, ** = p < 0.01, and *** = p < 0.001. The combination of TGFβ2 and H2O2 were compared with similar concentration of only-H2O2 and represented as # = p < 0.05, ## = p < 0.01, and ### = p < 0.001
The effect of only-TGFβ2, only-H2O2, and a combination of H2O2 and TGFβ2 on the expression of fibronectin is shown in Fig. 5. Immunofluorescence staining revealed that fibronectin was expressed both intracellularly as a diffused stain in the cytoplasm and extracellularly as a speckle of fibers. Fibronectin expression was found significantly increased in the treatment of TGFβ2 (10 ng/ml) and H2O2 (25 μM and 100 μM) compared to the respective concentration of only-H2O2 and compared to that of untreated cells.
Effects of H2O2 and TGFβ2 on fibronectin of the ARPE-19 cells (Immunofluorescence aspect). TGFβ2 exposure led to elongation of cell nuclei and increased expression of fibronectin. However, the combined exposure of H2O2 and TGFβ2 showed more significantly increased expression of fibronectin compared to untreated and only-H2O2 treated ARPE-19 cells
The results of protein expressions shown in Fig. 6. We have used ZO1 as epithelial and fibronectin as a mesenchymal marker. The combined effect of TGFβ2 and H2O2 on ZO1 remained less than that of the respective concentration of only-H2O2 treated cells. The fibronectin protein levels found increased by the simultaneous treatment of TGFβ2 (10 ng/ml) along with both 25 and 100 μM H2O2 treatment compared to that of untreated cells (# = p < 0.05, ## = p < 0.01, and ### = p < 0.001).
Effects of H2O2 and TGFβ2 on the expression of epithelial and epithelial–mesenchymal transition markers at the protein level in the ARPE-19 cells. The combined treatment of H2O2 and TGFβ2 showed reduced expression of ZO1 and showed increased expressions of fibronectin compared to untreated ARPE-19 cells and only-H2O2 treated cells. n = 5 for each group. Data are represented as a percentage of untreated cells as mean ± SEM. H2O2 alone and TGFβ2 alone exposure were compared with the untreated cells and shown with ns = non-significant, * = p < 0.05, ** = p < 0.01, and *** = p < 0.001. The combination of TGFβ2 and H2O2 compared with a similar concentration of only-H2O2 and represented as # = p < 0.05, ## = p < 0.01, and ### = p < 0.001
3.5 Effects on total glutathione content of TGFβ2 and H2O2 treated ARPE-19 cells
The results suggest the involvement of oxidative stress in the epithelial–mesenchymal transition of Retinal pigment epithelium cells (Fig. 7). The treatment of only-TGFβ2 (10 ng/ml) showed downregulated glutathione compared to untreated control cells (** = p < 0.01). The treatment of only-H2O2 treated cells (25 μM and 100 μM H2O2) also showed decreased glutathione ($ = p < 0.05, ** = p < 0.01). However, the simultaneous treatment of TGFβ2 (10 ng/ml) along with H2O2 (25 μM and 100 μM H2O2) showed significantly decreased glutathione compared to untreated control and only-TGFβ2 treated ARPE-19 cells (*** = p < 0.05, ### = p < 0.01).
Estimation of total glutathione of TGFβ2 and H2O2 treated ARPE-19 cells. The results suggested the involvement of oxidative stress in the epithelial–mesenchymal transition of retinal pigment epithelium cells. The treatment of only-TGFβ2 (10 ng/ml) showed downregulated glutathione compared to untreated control cells (** = p < 0.01). The treatment of only-H2O2 (25 μM and 100 μM H2O2) also showed decreased glutathione ($ = p < 0.05, ** = p < 0.01) compared to untreated control cells. However, the simultaneous treatment of TGFβ2 (10 ng/ml) and H2O2 (25 μM and 100 μM H2O2) showed significantly decreased glutathione compared to untreated control and only-TGFβ2 treated ARPE-19 cells (*** = p < 0.001, ### = p < 0.001)
4 Discussion
Network pharmacology is a computational approach to investigating the interactions of multiple proteins in various therapeutic conditions [49]. The results of the current study indicate that 123 (OS-DR) and 33 (OS-PVR) oxidative stress-associated proteins have a significant impact on the epithelial–mesenchymal transition of retinal pigment epithelium cells. KEGG pathway enrichment analysis further demonstrated that oxidative stress-associated proteins can activate pathways including PI3K-Akt, MAPK, and TGFβ-signaling. The literature further supports our findings by suggesting that ocular cells, particularly retinal pigment epithelium cells, undergo epithelial–mesenchymal transition predominantly through non-canonical TGFβ signaling, which includes the PI3K-Akt and MAPK/Erk signaling pathways. Hypoxia and inflammatory pathways also contribute to the execution of the epithelial–mesenchymal transition process in retinal pigment epithelium cells [50,51,52]. It is not known, however, how oxidative stress triggers SMAD and MEK/ERK signaling to increase TGFβ-mediated EMT. However, since TGFβ is stimulated by oxidative stress, SMAD activation may be brought on by TGFβ.
The reduced cell survival observed in the current study might be attributed to the increased oxidative stress in cells where the antioxidant capacity of retinal pigment epithelium cells is largely suppressed. Our findings of decreased viability of ARPE-19 cells are consistent with previous research in response to subtoxic concentrations (50–300 µM) of H2O2 [24, 53]. Oxidative stress has been shown to cause cell–cell dissociation, which may be required to initiate epithelial–mesenchymal transition [21, 54, 55]. α-SMA is an active isoform that is expressed specifically in myofibroblasts and is considered a critical marker for epithelial–mesenchymal transition [56]. According to the findings of a current study, the combined treatment of H2O2 and TGFβ2 upregulates the expression of α-SMA. Additionally, fibronectin is a key component of the extracellular matrix of drusen in oculopathies [57]. Results of the current study show that the level of fibronectin increases several-fold after the simultaneous treatment of TGFβ2 and H2O2. Our understanding suggests that the increase in fibronectin could be due to the induction of epithelial–mesenchymal transition in ARPE-19 cells under the influence of oxidative stress. Other mesenchymal markers, S100A4 and MMP9, are also upregulated epithelial markers E-cadherin, ZO1, Pax6, and β-catenin are downregulated as a result of the effects of oxidative stress. However, when only-H2O2 is exposed, Pax6 mRNA levels were found to be upregulated while S100A4 and MMP9 levels were downregulated, indicating the epithelial–mesenchymal transition of cells.
Earlier studies show that TGFβ is capable of raising ROS levels by suppressing the production of antioxidant enzymes and lowering the amounts of antioxidant enzymes [58]. Taken together with the results of the current study, the effect of only-H2O2 has a variable effect on the expression of various epithelial markers as well as mesenchymal markers, but combined treatments result in increased mesenchymal expression and decreased epithelial marker expression. As a result, the presence of TGFβ2 is required epithelial–mesenchymal transition of ARPE-19 cells; additionally, the presence of H2O2 enhances the effect of TGFβ2 and leads to a more significant increase in the expression of epithelial–mesenchymal transition. In the current study, we used total glutathione as an indicator of oxidative stress and our results indicate that the combined treatment of TGFβ2 and H2O2 resulted in a significant decrease in glutathione. In support of our results earlier study conducted by Wei and coworkers on lens epithelial cells showed that a low glutathione level is a key event in promoting epithelial–mesenchymal transition [20]. Furthermore, in addition to the liver, lungs, and lens epithelial cells, low glutathione levels have been seen in a number of human fibrotic diseases [20, 59,60,61].
In the current study, wound closure increased in response to oxidative stress when simultaneous treatment of H2O2 and TGFβ2 was given to ARPE-19 cells. Furthermore, the literature suggests that treatment of only-H2O2 is insufficient to induce epithelial–mesenchymal transition in cells [62]. Although, it is also suggested that only TGFβ2 treatment is insufficient to cause cell–cell junction dissociations [15]. As a result, the microenvironment containing both H2O2 and TGFβ2 appears to be required to induce the epithelial–mesenchymal transition of ARPE-19 cells, and further research is needed to understand the role of glutathione and the complex signaling cascade involved in the activation and induction of epithelial–mesenchymal transition of ARPE-19 cells under oxidative stress microenvironment.
In summary, the current work shows that mild oxidative stress can alter the epithelial–mesenchymal transitions of the retinal pigment epithelium in vitro and in-silico. According to this study, antioxidant medication may be used to treat epithelial–mesenchymal transitions of the retinal pigment epithelium. It is important to understand the complex interactions and roles of TGFβ2 and oxidative stress in the initiation, progression, and eventual discovery of potential therapeutics for anti-EMT treatment of epithelial–mesenchymal transitions of retinal pigment epithelium in humans. However, the first limitation of the current study is to elucidate the complex signaling cascade interplay and roles of TGFβ2 and oxidative stress in epithelial–mesenchymal transitions of the retinal pigment epithelium. The second limitation is that we only examined ARPE-19 cells in vitro; thus, the aforementioned results need to be confirmed using primary retinal pigment epithelial cells before being further tested in vivo. We currently intend to confirm it at the primary cell line level. The third limitation is that we have only used an in-silico (bioinformatics) method to clarify the significance of signaling cascades in the epithelial–mesenchymal transition of retinal pigment epithelial cells; however, we also need to validate this at the protein level using western blotting. The fourth limitation is that we only represented a 24-h exposure time since it is more physiologically relevant. It should be investigated to examine the effects of the treatment after 48 and 72 h. The fifth limitation is that glutathione was the only antioxidant enzyme used in the current study as a measure of oxidative stress; however, other antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) also need to be assessed for alterations.
5 Conclusions
Taken together, the results of the present study show that, the presence of hydrogen peroxide enhances TGFβ2-induced epithelial–mesenchymal transition of human retinal pigment epithelial cell line, ARPE-19 cells.
Availability of data and materials
Not applicable.
Abbreviations
- ARPE-19:
-
A human retinal pigment epithelial cell
- BCA:
-
Bicinchoninic acid assay
- BSA:
-
Bovine serum albumin
- cDNA:
-
Complementary DNA
- DABCO:
-
1,4-Diazabicyclo [2.2.2] octane
- DAPI:
-
4′,6-Diamidino-2-phenylindole
- DAVID:
-
Database annotation, visualization, and integrated discovery
- DMEM:
-
Dulbecco’s modified eagle medium
- DMSO:
-
Dimethyl sulfoxide
- DR:
-
Diabetic retinopathies
- DTNB:
-
5,5′-Dithiobis (2-nitrobenzoic acid)
- ECL:
-
Electrochemiluminescence
- EDTA:
-
Ethylenediamine tetraacetic acid
- EMT:
-
Epithelial–mesenchymal transition
- FBS:
-
Foetal bovine serum
- FoxO:
-
Forkhead box O
- GSH:
-
Glutathione
- H2O2 :
-
Hydrogen peroxide
- HIF-1:
-
Hypoxia-inducible factor
- HRP:
-
Horseradish peroxidase
- Jak-STAT:
-
Janus kinase/signal transducer and activator of transcription signaling pathway
- KEGG:
-
Kyoto encyclopaedia of genes and genomes
- MAPK:
-
Mitogen-activated protein kinase
- MMP9:
-
Matrix metallopeptidase 9
- MTT:
-
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide
- NF-kappa B:
-
Nuclear factor kappa B
- PBS:
-
Phosphate buffered saline
- PCR:
-
Polymerase chain reaction
- PDR:
-
Proliferative diabetic retinopathy
- PI3K-Akt:
-
Phosphoinositide-3-kinase
- PPI:
-
Protein–protein interaction
- PVR:
-
Proliferative vitreoretinopathy
- ROS:
-
Reactive oxygen species
- RPE cells:
-
Retinal pigment epithelial cells
- SDS:
-
Sodium dodecyl sulfate
- SEM:
-
Standard error of means
- TBS:
-
Tris-buffered saline
- TBST:
-
Tris-buffered saline with 0.1% Tween® 20 detergent
- TGFβ2:
-
Transforming growth factors-β2
- TNF:
-
Tumor necrosis factor
- VEGF:
-
Vascular endothelial growth factor
- ZO1:
-
Zonula occludens-1
- α-SMA:
-
Alpha-smooth muscle actin
References
Panda-Jonas S, Jonas JB, Jakobczyk-Zmija M (1996) Retinal pigment epithelial cell count, distribution, and correlations in normal human eyes. Am J Ophthalmol 121:181–189. https://doi.org/10.1016/S0002-9394(14)70583-5
Zhou M, Geathers JS, Grillo SL, Weber SR, Wang W, Zhao Y, Sundstrom JM (2020) Role of epithelial–mesenchymal transition in retinal pigment epithelium dysfunction. Front Cell Dev Biol 8:501. https://doi.org/10.3389/fcell.2020.00501
Gelat B, Rathaur P, Malaviya P, Patel B, Trivedi K, Johar K, Gelat R (2022) The intervention of epithelial–mesenchymal transition in homeostasis of human retinal pigment epithelial cells: a review. J Histotechnol 45:148–160. https://doi.org/10.1080/01478885.2022.2137665
Rizzolo LJ (2007) Development and Role of tight junctions in the retinal pigment epithelium. Int Rev Cytol 258:195–234. https://doi.org/10.1016/S0074-7696(07)58004-6
Xia T, Rizzolo LJ (2017) Effects of diabetic retinopathy on the barrier functions of the retinal pigment epithelium. Vision Res 139:72–81. https://doi.org/10.1016/j.visres.2017.02.006
Narimatsu T, Ozawa Y, Miyake S, Kubota S, Hirasawa M, Nagai N, Shimmura S, Tsubota K (2013) Disruption of cell-cell junctions and induction of pathological cytokines in the retinal pigment epithelium of light-exposed mice. Investig Ophthalmol Vis Sci 54:4555–4562. https://doi.org/10.1167/iovs.12-11572
Acloque H, Adams MS, Fishwick K, Bronner-Fraser M, Nieto MA (2009) Epithelial–mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest 119:1438–1449. https://doi.org/10.1172/JCI38019
Kalluri R, Weinberg RA (2009) The basics of epithelial–mesenchymal transition. J Clin Invest 119:1420–1428. https://doi.org/10.1172/JCI39104
Iizuka D, Sasatani M, Barcellos-Hoff MH, Kamiya K (2017) Hydrogen peroxide enhances tgf-mediated epithelial-to-mesenchymal transition in human mammary epithelial mcf-10a cells. Anticancer Res 37:987–995. https://doi.org/10.21873/anticanres.11408
Zhang Y, Wang K, Pan J, Yang S, Yao H, Li M, Li H, Lei H, Jin H, Wang F (2020) Exosomes mediate an epithelial–mesenchymal transition cascade in retinal pigment epithelial cells: implications for proliferative vitreoretinopathy. J Cell Mol Med 24:13324–13335. https://doi.org/10.1111/jcmm.15951
Kalluri R, Neilson EG (2003) Epithelial–mesenchymal transition and its implications for fibrosis. J Clin Invest 112:1776–1784. https://doi.org/10.1172/JCI200320530
Liang CM, Tai MC, Chang YH, Chen YH, Chen CL, Lu DW, Chen JT (2011) Glucosamine inhibits epithelial-to-mesenchymal transition and migration of retinal pigment epithelium cells in culture and morphologic changes in a mouse model of proliferative vitreoretinopathy. Acta Ophthalmol 89:505–514. https://doi.org/10.1111/j.1755-3768.2011.02147.x
Xu S, Zhan M, Wang J (2017) Epithelial-to-mesenchymal transition in gallbladder cancer: from clinical evidence to cellular regulatory networks. Nat Publ Gr. https://doi.org/10.1038/cddiscovery.2017.69
Nisticò P, Bissell MJ, Radisky DC (2012) Epithelial–mesenchymal transition: general principles and pathological relevance with special emphasis on the role of matrix metalloproteinases. Cold Spring Harb Perspect Biol. https://doi.org/10.1101/cshperspect.a011908
Yang IH, Lee JJ, Wu PC, Kuo HK, Kuo YH, Huang HM (2020) Oxidative stress enhanced the transforming growth factor-β2-induced epithelial–mesenchymal transition through chemokine ligand 1 on ARPE-19 cell. Sci Rep 10:1–10. https://doi.org/10.1038/s41598-020-60785-x
Tamiya S, Kaplan HJ (2016) Role of epithelial–mesenchymal transition in proliferative vitreoretinopathy. Exp Eye Res 142:26–31. https://doi.org/10.1016/j.exer.2015.02.008
Ghosh S, Shang P, Terasaki H, Stepicheva N, Hose S, Yazdankhah M, Weiss J, Sakamoto T, Bhutto IA, Xia S, Zigler JS, Kannan R, Qian J, Handa JT, Sinha D (2018) A role for βA3/A1-crystallin in type 2 EMT of RPE cells occurring in dry age-related macular degeneration. Investig Ophthalmol Vis Sci 59:AMD104–AMD113. https://doi.org/10.1167/iovs.18-24132
Ishikawa K, He S, Terasaki H, Nazari H, Zhang H, Spee C, Kannan R, Hinton DR (2015) Resveratrol inhibits epithelial–mesenchymal transition of retinal pigment epithelium and development of proliferative vitreoretinopathy. Sci Rep. https://doi.org/10.1038/srep16386
Ung et al (2017) Oxidative stress and reactive oxygen species: a review of their role in ocular disease. Clin Sci 131:2865–2883. https://doi.org/10.1042/CS20171246
Wei Z, Caty J, Whitson J, Zhang AD, Srinivasagan R, Kavanagh TJ, Yan H, Fan X (2017) Reduced glutathione level promotes epithelial–mesenchymal transition in lens epithelial cells via a Wnt/β-catenin–mediated pathway: relevance for cataract therapy. Am J Pathol 187:2399–2412. https://doi.org/10.1016/j.ajpath.2017.07.018
Inumaru J, Nagano O, Takahashi E, Ishimoto T, Nakamura S, Suzuki Y, Niwa SI, Umezawa K, Tanihara H, Saya H (2009) Molecular mechanisms regulating dissociation of cell–cell junction of epithelial cells by oxidative stress. Genes Cells 14:703–716. https://doi.org/10.1111/j.1365-2443.2009.01303.x
Mateos MV (2015) Inflammation and oxidative stress in retinal diseases: the role of intracellular signaling in the retinal pigment epithelium. Int J Ophthalmol Clin Res. https://doi.org/10.23937/2378-346x/1410033
Cano M, Wang L, Wan J, Barnett BP, Ebrahimi K, Qian J, Handa JT (2014) Oxidative stress induces mitochondrial dysfunction and a protective unfolded protein response in RPE cells. Free Radic Biol Med 69:1–14. https://doi.org/10.1038/jid.2014.371
Angulo Daniela F (2015) Intracellular redox status and cell death Induced by H2O2 in a human retinal epithelial cell line (ARPE-19). Am J Biosci. https://doi.org/10.11648/j.ajbio.20150303.15
Kim MH, Chung J, Wook Yang J, Chung SM, Kwag NH, Yoo JS (2003) Hydrogen peroxide-induced cell death in a human retinal pigment epithelial cell line, ARPE-19. Korean J Ophthalmol 17:19–28. https://doi.org/10.3341/kjo.2003.17.1.19
Sen CK, Khanna S, Gordillo G, Bagchi D, Bagchi M, Roy S (2002) Oxygen, oxidants, and antioxidants in wound healing: an emerging paradigm. Ann N Y Acad Sci. https://doi.org/10.1111/j.1749-6632.2002.tb02920.x
Sjølie AK, Klein R, Porta M, Orchard T, Fuller J, Parving HH, Bilous R, Aldington S, Chaturvedi N (2011) Retinal microaneurysm count predicts progression and regression of diabetic retinopathy. Post-hoc results from the DIRECT Programme. Diabet Med 28:345–351. https://doi.org/10.1111/j.1464-5491.2010.03210.x
Shukal D, Bhadresha K, Shastri B, Mehta D, Vasavada A, Johar K Sr (2020) Dichloroacetate prevents TGFβ-induced epithelial–mesenchymal transition of retinal pigment epithelial cells. Exper Eye Res 197:108072
Cao Q, Deji QZ, Liu YJ, Ye W, Zhaba WD, Jiang Q, Yan F (2019) The role of mechanical stretch and TGF-β2 in epithelial–mesenchymal transition of retinal pigment epithelial cells. Int J Ophthalmol 12:1832–1838. https://doi.org/10.18240/ijo.2019.12.03
Akhurst RJ, Hata A (2012) Targeting the TGFβ signalling pathway in disease. Nat Rev Drug Discov 11:790–811. https://doi.org/10.1038/nrd3810
Krstić J, Trivanović D, Mojsilović S, Santibanez JF (2015) Transforming growth factor-beta and oxidative stress interplay: implications in tumorigenesis and cancer progression. Oxid Med Cell Longev. https://doi.org/10.1155/2015/654594
Liu R, FRB KP (2010) and medicine, undefined 2010, Oxidative stress and glutathione in TGF-β-mediated fibrogenesis, Elsevier. (n.d.). https://www.sciencedirect.com/science/article/pii/S0891584909005681. Accessed 31 July 2023
Wu WS (2006) The signaling mechanism of ROS in tumor progression. Cancer Metastasis Rev 25:695–705. https://doi.org/10.1007/s10555-006-9037-8
Parapuram SK, Chang B, Li L, Hartung RA, Chalam KV, Nair-Menon JU, Hunt DM, Hunt RC (2009) Differential effects of TGFβ and vitreous on the transformation of retinal pigment epithelial cells. Investig Ophthalmol Vis Sci 50:5965–5974. https://doi.org/10.1167/iovs.09-3621
Mony S, Lee SJ, Harper JF, Barwe SP, Langhans SA (2013) Regulation of Na, K-ATPase β1-subunit in TGF-β2-mediated epithelial-to-mesenchymal transition in human retinal pigmented epithelial cells. Exp Eye Res 115:113–122. https://doi.org/10.1016/j.exer.2013.06.007
Tosi GM, Caldi E, Neri G, Nuti E, Marigliani D, Baiocchi S, Traversi C, Cevenini G, Tarantello A, Fusco F, Nardi F, Orlandini M, Galvagni F (2017) HTRA1 and TGF-β1 concentrations in the aqueous humor of patients with neovascular age-related macular degeneration. Investig Ophthalmol Vis Sci 58:162–167. https://doi.org/10.1167/iovs.16-20922
Saika S (2006) TGFβ pathobiology in the eye. Lab Investig 86:106–115. https://doi.org/10.1038/labinvest.3700375
Shu DY, Lovicu FJ (2017) Myofibroblast transdifferentiation: the dark force in ocular wound healing and fibrosis. Prog Retin Eye Res 60:44–65. https://doi.org/10.1016/j.preteyeres.2017.08.001
Liu RM, Desai LP (2015) Reciprocal regulation of TGF-β and reactive oxygen species: a perverse cycle for fibrosis. Redox Biol 6:565–577. https://doi.org/10.1016/j.redox.2015.09.009
Stelzer G, Rosen N, Plaschkes I, Zimmerman S, Twik M, Fishilevich S, Iny Stein T, Nudel R, Lieder I, Mazor Y, Kaplan S, Dahary D, Warshawsky D, Guan-Golan Y, Kohn A, Rappaport N, Safran M, Lancet D (2016) The GeneCards suite: from gene data mining to disease genome sequence analyses. Curr Protoc Bioinforma 2016:1.30.1-1.30.33. https://doi.org/10.1002/cpbi.5
Piñero J, Ramírez-Anguita JM, Saüch-Pitarch J, Ronzano F, Centeno E, Sanz F, Furlong LI (2020) The DisGeNET knowledge platform for disease genomics: 2019 update. Nucleic Acids Res 48:D845–D855. https://doi.org/10.1093/nar/gkz1021
Szklarczyk D, Gable AL, Nastou KC, Lyon D, Kirsch R, Pyysalo S, Doncheva NT, Legeay M, Fang T, Bork P, Jensen LJ, von Mering C (2021) The STRING database in 2021: customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res 49:D605–D612. https://doi.org/10.1093/nar/gkaa1074
Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T (2003) Cytoscape: a software Environment for integrated models of biomolecular interaction networks. Genome Res 13:2498–2504. https://doi.org/10.1101/gr.1239303
Jiao X, Sherman BT, Huang DW, Stephens R, Baseler MW, Lane HC, Lempicki RA (2012) DAVID-WS: a stateful web service to facilitate gene/protein list analysis. Bioinformatics 28:1805–1806. https://doi.org/10.1093/bioinformatics/bts251
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63. https://doi.org/10.1016/0022-1759(83)90303-4
Shukal D, Bhadresha K, Shastri B, Mehta D, Vasavada A, Johar K (2020) Dichloroacetate prevents TGFβ-induced epithelial–mesenchymal transition of retinal pigment epithelial cells. Exp Eye Res. https://doi.org/10.1016/j.exer.2020.108072
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Rahman I, Kode A, Biswas SK (2007) Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat Protoc 1:3159–3165. https://doi.org/10.1038/nprot.2006.378
Chandran U, Mehendale N, Patil S, Chaguturu R, Patwardhan B (2017) Network pharmacology. Innov Approac Drug Discov Ethnopharmacol Syst Biol Holist Target. https://doi.org/10.1016/B978-0-12-801814-9.00005-2
Parvathaneni K, Grigsby JG, Betts BS, Tsin AT (2013) Estrogen-induced retinal endothelial cell proliferation: possible involvement of pigment epithelium-derived factor and phosphoinositide 3-kinase/mitogen-activated protein kinase pathways. J Ocul Pharmacol Ther 29:27–32. https://doi.org/10.1089/jop.2011.0252
Bayan N, Yazdanpanah N, Rezaei N (2022) Role of toll-like receptor 4 in diabetic retinopathy. Pharmacol Res. https://doi.org/10.1016/j.phrs.2021.105960
Min J, Zeng T, Roux M, Lazar D, Chen L, Tudzarova S (2021) The role of HIF1α-PFKFB3 pathway in diabetic retinopathy. J Clin Endocrinol Metab 106:2505–2519. https://doi.org/10.1210/clinem/dgab362
Yang Wang Z, Jun Shen L, Tu LL, Ning Hu D, Liu GY, Lou Zhou Z, Lin Y, Chen LH, Qu J (2009) Erythropoietin protects retinal pigment epithelial cells from oxidative damage. Free Radic Biol Med 46:1032–1041. https://doi.org/10.1016/j.freeradbiomed.2008.11.027
Yang X, Chung JY, Rai U, Esumi N (2018) Cadherins in the retinal pigment epithelium (RPE) revisited: P-cadherin is the highly dominant cadherin expressed in human and mouse RPE in vivo. PLoS ONE 13:1–20. https://doi.org/10.1371/journal.pone.0191279
Chen X, Yan H, Chen Y, Li G, Bin Y, Zhou X (2021) Moderate oxidative stress promotes epithelial–mesenchymal transition in the lens epithelial cells via the TGF-β/Smad and Wnt/β-catenin pathways. Mol Cell Biochem 476:1631–1642. https://doi.org/10.1007/s11010-020-04034-9
He H, Kuriyan AE, Su CW, Mahabole M, Zhang Y, Zhu YT, Flynn HW, Parel JM, Tseng SCG (2017) Inhibition of proliferation and epithelial mesenchymal transition in retinal pigment epithelial cells by heavy chain-hyaluronan/pentraxin 3. Sci Rep. https://doi.org/10.1038/srep43736
Biasella F, Plössl K, Karl C, Weber BHF, Friedrich U (2020) Altered protein function caused by AMD-associated variant rs704 links vitronectin to disease pathology. Investig Ophthalmol Vis Sci. https://doi.org/10.1167/IOVS.61.14.2
Liu RM, Gaston Pravia KA (2010) Oxidative stress and glutathione in TGF-β-mediated fibrogenesis. Free Radic Biol Med 48:1–15. https://doi.org/10.1016/j.freeradbiomed.2009.09.026
Roum JH, Buhl R, McElvaney NG, Borok Z, Crystal RG (1993) Systemic deficiency of glutathione in cystic fibrosis. J Appl Physiol 75:2419–2424. https://doi.org/10.1152/jappl.1993.75.6.2419
Bianchi G, Bugianesi E, Ronchi M, Fabbri A, Zoli M, Marchesini G (1997) Glutathione kinetics in normal man and in patients with liver cirrhosis. J Hepatol 26:606–613. https://doi.org/10.1016/S0168-8278(97)80426-6
Deger Y, Yur F, Ertekin A, Mert N, Dede S, Mert H (2007) Protective effect of α-tocopherol on oxidative stress in experimental pulmonary fibrosis in rats. Cell Biochem Funct 25:633–637. https://doi.org/10.1002/cbf.1362
Felton VM, Borok Z, Willis BC (2009) N-acetylcysteine inhibits alveolar epithelial–mesenchymal transition. Am J Physiol Lung Cell Mol Physiol. https://doi.org/10.1152/ajplung.00009.2009
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Writing—original draft by BG, PR, PM, BP and KJ. KJ and AV formulated the hypothesis. BG, PM, BP and PR were responsible for conducting the experiments. All authors have contributed in the writing of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable.
Competing interests
The authors have declared that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gelat, B., Rathaur, P., Malaviya, P. et al. Hydrogen peroxide enhances transforming growth factor beta-2 induced epithelial–mesenchymal transition of ARPE-19 cells. Beni-Suef Univ J Basic Appl Sci 12, 102 (2023). https://doi.org/10.1186/s43088-023-00421-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43088-023-00421-5