Abstract
Background
Metabolic-associated fatty liver disease (MAFLD) has emerged as the predominant form of chronic liver disease globally linked with heightened cardiovascular disease (CVD) risk, the leading cause of mortality among affected individuals.
Aim
This study aims to assess serum PTX3 (pentraxin 3) and platelet-derived growth factor receptor beta (PDGFRβ) as potential non-invasive biomarkers for predicting cardiovascular risk (CVR) in MAFLD patients.
Method
A case–control investigation encompassing 84 MAFLD patients without prior CVD history and 30 age- and gender-matched healthy controls was conducted. Both cohorts underwent comprehensive laboratory and radiological evaluations. CVR was evaluated through common carotid artery intima-media thickness (IMT), Framingham risk score, and QRISK 2 score. The efficacy of two ELISA biomarkers PTX3 and PDGFRβ was examined for correlation with CVR in MAFLD patients.
Results
MAFLD patients displayed significantly heightened levels of PTX3 and PDGFβ compared to healthy controls (P < 0.001, P = 0.016, respectively). PDGFβ exhibited a notably positive correlation with the Framingham score (P = 0.016), while no significant correlation was observed with pentraxin 3 (P = 0.061). Univariate and multivariate analyses identified diabetes mellitus (DM) (P < 0.001*), hypertension (P = 0.005), visceral fat (P < 0.001*), waist/hip circumference (P = 0.04), and PDGFβ (P = 0.03) as robust predictors of CVR, with PTX3 demonstrating limited prognostic utility.
Conclusion
PDGFβ emerged as a promising early non-invasive predictor of CVR in MAFLD patients, highlighting its potential role in guiding tailored preventive interventions, while PTX3 exhibited a modest impact warranting further investigation.
Similar content being viewed by others
Introduction
The escalating prevalence of NAFLD is intimately connected to the rising occurrence of metabolic disorders [1]. This intricate relationship not only imposes a significant strain on public health systems but also heightens the susceptibility to cardiovascular complications, thereby exacerbating both morbidity and mortality [2]. Recently, the term “Metabolic-Associated Steatotic Liver Disease” (MAFLD) has been proposed within the context of NAFLD to better encapsulate the multifaceted nature of this condition [3]. Beyond its hepatic manifestations, MAFLD is acknowledged as a systemic ailment with profound implications, particularly regarding cardiovascular risk (CVR) [2]. Individuals afflicted with MAFLD face an augmented likelihood of cardiovascular events, emphasizing the urgency for effective risk assessment tools [3].
Pentraxin-3 (PTX3) is a member of the pentraxin superfamily akin to acute-phase reactants like C-reactive protein (CRP) [4]. It serves as a versatile soluble recognition receptor, exerting influence on the immunoinflammatory response [5]. Its production is induced by pro-inflammatory stimuli such as tumor necrosis factor-alpha (TNF-α), interleukin-1β (IL-1β), and lipopolysaccharides (LPS), all recognized as pivotal factors in the development of non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH) [6]. Notably, PTX3 has been implicated in the pathogenesis of atherosclerosis progression and cardiovascular events, suggesting a possible role in cardiovascular risk assessment [7]. Furthermore, elevated PTX3 levels have been linked to impaired endothelial function, contributing to cardiovascular risk across various clinical scenarios. Numerous studies have proposed that heightened PTX3 may serve as a biomarker for predicting cardiovascular risk and prognosis in patients with diverse cardiovascular conditions [4, 8, 9].
Hepatic stellate cell activation has been associated with platelet-derived growth factor receptor beta (PDGFRβ), which has been the focus of numerous therapeutic investigations, particularly in MAFLD patients [10]. PDGFβ has been implicated in the pathogenesis of atherosclerosis, vascular remodeling, and endothelial dysfunction [11,12,13]. While existing studies offer insights into the potential roles of PTX3 and PDGFβ in cardiovascular risk, further research is warranted to specifically explore the relationship between PDGFβ levels and cardiovascular outcomes in MAFLD patients. Unraveling the mechanisms by which PDGF-BB contributes to cardiovascular risk in this population could unveil novel therapeutic targets for averting cardiovascular complications in MAFLD patients.
This clinical study aims to address the pressing need for non-invasive biomarkers capable of robustly predicting CVR in MAFLD patients. PTX3 and PDGFβ have emerged as promising candidates, reflecting the intricate interplay between hepatic dysfunction, chronic inflammation, and cardiovascular complications.
Patients and methods
Study design
This prospective case–control study aimed to investigate the utility of PTX3 and platelet-derived growth factor receptor beta (PDGFβ) as non-invasive biomarkers for predicting CVR in patients diagnosed with MAFLD [2]. The study adheres to ethical guidelines and has received approval from the Institutional Review Board (IRB).
Study participants
The study population comprised two groups
Group I includes individuals diagnosed with MAFLD, while Group II consists of healthy controls without MAFLD. Participants in Group I were recruited from hepatology and gastroenterology clinics, National Liver Institute Hospital (NLI), Menoufia University, Egypt. Institutional review board of NLI approval and written informed patients’ consent are prerequisites for enrolment in this study. Inclusion criteria encompass age (18–70 years) with a confirmed diagnosis of MAFLD [2]. Group II participants were with matched age and sex.
Exclusion criteria
The exclusion criteria were as follows:
-
Age less than 18
-
Other causes of liver chronic diseases (viral, autoimmune, and metabolic)
-
Alcohol consumption
-
≥ 10% weight loss within 6 months
-
Patients with a previous history of cardiovascular events (angina, myocardial infarction, transient ischemic attack, and stroke)
-
Patients receiving drugs that cause fatty liver such as amiodarone, diltiazem, tamoxifen, and steroids
-
Patients who use statins
-
Patients with liver decompensation, hepatic encephalopathy, ascites, and variceal bleeding
-
Patients with chronic kidney diseases, systemic autoimmune diseases, and sepsis
-
Patients suggested to have urinary tract infections (UTI), gynecological disorders, or neurological disorders as PTX3 is overexpressed in these disorders
Sample size and sampling
The sample size calculation was based on the prevalence of MAFLD and the anticipated effect size for the biomarkers. A total of 114 participants were included: 84 MAFLD patients (Group I) and 30 healthy controls (Group II). The participants were selected using a convenience sampling method from the hepatology and gastroenterology clinics at the National Liver Institute Hospital (NLI), Menoufia University, Egypt.
Clinical and laboratory assessment
All participants underwent a thorough clinical evaluation, including a detailed medical history and physical examination. Anthropometric measurements, including weight, height, waist circumference, and hip circumference, were recorded. Laboratory assessments included liver function tests, lipid profiles, fasting blood glucose, and insulin levels. Inflammatory markers such as high-sensitivity C-reactive protein (hs-CRP) were also measured.
Abdominal ultrasonography to confirm the diagnosis of MAFLD and to assess liver steatosis.
Biomarker measurement
Plasma PTX3 was measured using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (Quantikine) (R&D Systems, Inc. 614 McKinley Place NE Minneapolis, MN 55413, USA) [14].
Plasma PDGFβ was measured with a commercially available ELISA kit (ThermoFisher Scientific), according to the manufacturer’s instructions.
All plasma samples were diluted 1/10 with diluent provided by the manufacturer. Absorbance values were obtained with an iMark™ microplate absorbance reader (Bio-Rad) [15]. Blood samples were collected after an overnight fast, centrifuged, and stored at − 80 °C until analysis. The ELISA procedures were performed according to the manufacturer’s instructions.
Cardiovascular risk parameters
Common carotid artery intima-media thickness (IMT) was measured using B-mode ultrasonography as a surrogate marker for subclinical atherosclerosis and cardiovascular risk (CVR) [16], non-invasive scores (Framingham risk score, which is based on age, sex, total cholesterol, smoking, HDL, systolic blood pressure, BP being treated with antihypertensive) [17], all were defined as CVD risk predictors at 10 years. Individuals with low risk have 10% or less CHD risk at 10 years, with intermediate risk 10–20%, and with high risk 20% or more.
Statistical analysis
Data were analyzed using SPSS software version 26.0. Continuous variables were expressed as mean ± standard deviation (SD), and categorical variables were expressed as frequencies and percentages. Independent t-tests and chi-square tests were used to compare continuous and categorical variables, respectively, between the two groups. Pearson’s correlation coefficient was employed to assess the relationship between PTX3, PDGFβ, and CVR parameters. Multivariate regression analysis was conducted to identify independent predictors of CVR. A P value of < 0.05 was considered statistically significant.
Results
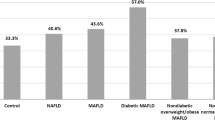
There is no significant difference in demographics between the two groups. Clinically, fatty liver patients (Group I) show a significantly higher prevalence of diabetes (DM) and hypertension (HTN) compared to healthy controls (Group II) (Table 1). Pentraxin 3 and PDGFβ exhibited statistically significant differences between the two groups (Table 2). Pentraxin 3 and PDGFβ show significant correlations with various parameters such as age, intimal thickness (IMT), waist/hip ratio, and BMI. Some correlations are positive (e.g., with age), while others are negative (e.g., with HDL) (Table 3). According to a Framingham score of more than 10, univariate analysis revealed that age, DM, HTN, waist/hip ratio, and PDGFβ are identified as significant factors affecting CVR in NAFLD patients, and the results of multivariate analysis highlight the importance of considering age, diabetes, visceral fat level, and PDGFβ in assessing cardiovascular risk in MAFLD patients (Table 4). ROC curves of PTX3 and in predicting CVR are illustrated in Fig. 1 with higher accuracy of PDGFβ. Univariate and multivariate Logistic regression analysis for cardiovascular risk predictors in MAFLD patients had defined age, DM, and PDGFβ with OR 1.148, 1.929, and 1.263, respectively (Table 5).
Discussion
Understanding the role of PTX3 and PDGFβ in predicting cardiovascular risk in MAFLD patients is crucial, not only for refining risk assessment strategies but also for facilitating targeted interventions.
Examining pentraxin 3 levels in MAFLD patients compared to healthy controls revealed significant differences, with implications for understanding potential cardiovascular risk in this vulnerable population. Pentraxin 3 levels ranged from 0.50 to 12.20, with a mean of 2.92 ± 2.45, while in controls, the range was narrower, varying from 0.50 to 6.0, with a mean of 1.73 ± 1.18 (P < 0.001). Previous research has associated PTX3 as an inflammatory marker with elevated levels in NAFLD patients, especially in those with advanced fibrosis [7, 18, 19]. These findings, combined with our study, highlight the potential role of PTX3 as a non-invasive marker in MAFLD patients.
Pentraxin 3 (PTX3) belongs to the pentraxin family of proteins involved in the inflammatory response and has implications in cardiovascular diseases [20]. Remarkably, in the current study, correlation studies revealed significant associations of PTX3 with cardiovascular risk factors and scores, including waist/hip circumference, and intimal thickening by echocardiography.
Literature reports suggest that in patients with NAFLD, PTX3 levels are linked to visceral fat accumulation and cardiovascular risk. Studies have shown that PTX3 levels are significantly elevated in NAFLD patients with visceral obesity, often reflected by increased waist circumference, compared to those without visceral obesity [21]. Additionally, higher PTX3 levels are associated with an increased risk of developing cardiovascular complications in these patients [22]. Moreover, PTX3 levels correlate positively with visceral fat accumulation and insulin resistance in patients with NAFLD, indicating a relationship between waist/hip circumference, PTX3 levels, and cardiovascular risk in NAFLD patients [23].
Investigating PDGFβ in our study revealed elevated levels in MAFLD patients compared to healthy controls (P 0.016). Previous research suggests a role for PDGFβ in NAFLD-related fibrosis, with further validation of fibrosis scores containing PDGFβ, such as the PRTA score, demonstrating efficiency in grading fibrosis in NAFLD cases [10].
Our study established a positive correlation of PDGFβ with age, BMI, intimal thickening by echocardiography, systolic blood pressure, and Framingham score. Age, BMI, and elevated systolic blood pressure are well-established risk factors for cardiovascular disease, and their associations with PDGFβ further support its potential role in predicting cardiovascular risk in MAFLD patients [24].
Notably, the correlation between both biomarkers in our study and various clinical and metabolic cardiovascular risk factors highlights their potential as cardiovascular risk predictors in NAFLD patients. PDGFβ appears to be a more efficient predictor of cardiovascular risk due to its intimate relationship with high-risk predictors compared to PTX3.
Regression analysis negated any role for PTX3 in predicting cardiovascular risk, while PDGFβ showed significance (P 0.03). Additionally, DM, hypertension, visceral fat, and waist/hip circumference proved efficacy in predicting cardiovascular risk in MAFLD cases.
Despite these findings, analysis of the ROC curves revealed slightly higher sensitivity for PDGFβ (69.39%) compared to PTX3 (63.27%), with relatively similar positive predictive values. These results emphasize the complex nature of predicting Framingham risk using inflammatory markers, highlighting the need for comprehensive risk assessment in individuals with diabetes and high BMI [25].
Endorsing PDGFβ in cardiovascular risk scores could be valuable in MAFLD cases, necessitating further research for enhanced prediction capabilities. Further studies in larger and more diverse populations are necessary to validate these findings.
Conclusion
Our study underscores the potential of PDGFβ as a non-invasive biomarker for predicting cardiovascular risk in MAFLD patients while highlighting the limited role of PTX3 in this context. Integrating PDGFβ, along with traditional risk factors, could improve risk stratification and inform targeted interventions for managing cardiovascular risk in MAFLD. Further validation studies in diverse populations are warranted to establish the clinical significance of these biomarkers in routine practice.
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
Abbreviations
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- BMI:
-
Body mass index
- CRP:
-
C-reactive protein
- FB:
-
Fasting blood sugar
- PTX3:
-
Pentraxin 3
- HSCRP:
-
High-sensitivity C-reactive protein
- IMT:
-
Intima-media thickness
- CVR:
-
Cardiovascular risk
- HDL:
-
High-density lipoprotein
- LDL:
-
Low-density lipoprotein
- NAFLD:
-
Non-alcoholic fatty liver disease
- MAFLD:
-
Metabolic-associated fatty liver disease
- MASLD:
-
Metabolic-associated steatotic liver disease
- PPBG:
-
Postprandial blood glucose
- TG:
-
Triglycerides
- IMT:
-
Intimal thickness
- HTN:
-
Hypertension
- QR risk:
-
Quantitative risk of stroke
- QUIKI:
-
Quantitative Insulin Sensitivity Check Index
- HOMA:
-
Homeostatic Model Assessment
- PDGFβ:
-
Platelet-derived growth factor
- DM:
-
Diabetes mellitus
References
Iqbal U, Perumpail BJ, Akhtar D, Kim D, Ahmed A (2019) The epidemiology, risk profiling and diagnostic challenges of nonalcoholic fatty liver disease. Medicines. 6(1):41. https://doi.org/10.3390/medicines6010041. (PMID: 30889791; PMCID: PMC6473603)
Platek AE, Szymanska A (2023) Metabolic dysfunction-associated steatotic liver disease as a cardiovascular risk factor. Clin Exp Hepatol. 9(3):187–192. https://doi.org/10.5114/ceh.2023.130744. (Epub 2023 Aug 28. PMID: 37790680; PMCID: PMC10544058)
Duell PB, Welty FK, Miller M, Chait A, Hammond G, Ahmad Z, Cohen DE, Horton JD, Pressman GS, Toth PP, American Heart Association Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Hypertension; Council on the Kidney in Cardiovascular Disease; Council on Lifestyle and Cardiometabolic Health; and Council on Peripheral Vascular Disease (2022) Nonalcoholic fatty liver disease and cardiovascular risk: a scientific statement from the American Heart Association. Arterioscler Thromb Vasc Biol 42(6):e168–e185. https://doi.org/10.1161/ATV.0000000000000153. (Epub 2022 Apr 14. PMID: 35418240)
Norata GD, Garlanda C, Catapano AL (2010) The long pentraxin PTX3: a modulator of the immunoinflammatory response in atherosclerosis and cardiovascular diseases. Trends Cardiovasc Med 20:35–40
Mantovani A, Garlanda C, Battazzi B (2003) Pentraxin 3, a nonredundant soluble pattern recognition receptor involved in innate immunity. Vaccine 21:S43–S47
Muller B, Peri G, Doni A, Torri V, Landmann R, Bottazzi B, Mantovani A (2001) Circulating levels of the long pentraxin PTX3 correlate with severity of infection in critically ill patients. Crit Care Med 29(7):1404–1407. https://doi.org/10.1097/00003246-200107000-00017. (PMID: 11445697)
Makhlouf M, Saleh S, Rushdy M, Abdelhakam S, Abd-Elgani E (2019) Pentraxin-3 in non-alcoholic fatty liver disease and its affection by concomitant chronic hepatitis C infection. Egypt Liver Journal 9:7. https://doi.org/10.1186/s43066-019-0009-4
Salio M, Chimenti S, de Angelis N, Molla F, Maina V, Nebuloni M, Pasqualini F, Latini RA, Garlanda C, Mantovani A (2008) Cardioprotective function of the long pentraxin PTX3 in acute myocardial infarction. Circulation 117:1055–1064
Jenny NS, Arnold AM, Kuller LH, Tracy RP, Psaty BM (2009) Associations of pentraxin 3 with cardiovascular disease and all-cause death: the Cardiovascular Health Study. Arterioscler Thromb Vasc Biol 29(4):594–599
Lambrecht J, Verhulst S, Mannaerts I, Sowa JP, Best J, Canbay A, Reynaert H, van Grunsven LA (2019) A PDGFRβ-based score predicts significant liver fibrosis in patients with chronic alcohol abuse. NAFLD and viral liver disease. EBioMedicine. 43:501–512. https://doi.org/10.1016/j.ebiom.2019.04.036. (Epub 2019 Apr 27. PMID: 31036530; PMCID: PMC6558023.)
Raines EW (2004) PDGF and cardiovascular disease. Cytokine Growth Factor Rev 15(4):237–254
Lindahl P, Johansson BR, Levéen P, Betsholtz C (1997) Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science 277(5326):242–245
Antoniades C, Shirodaria C, Warrick N, Cai S, de Bono J, Lee J, et al. 5-methyltetrahydrofolate rapidly improves endothelial function and decreases superoxide production in human vessels: effects on vascular tetrahydrobiopterin availability and endothelial nitric oxide synthase coupling. Circulation. 2006 Sep 12;114[11]:1193–201. https://doi.org/10.1161/CIRCULATIONAHA.106.612325. Epub 2006 Aug 28. PMID: 16940192.Human pentraxin 3/TSG-14 ELISA-quantikine PTX30B: R&D Systems [rndsystems.com]
Keskiner I, Lutfioğlu M, Aydogdu A, Saygun NI, Serdar MA. Effect of Photobiomodulation on Transforming Growth Factor-β1, Platelet-Derived Growth Factor-BB, and Interleukin-8 Release in Palatal Wounds After Free Gingival Graft Harvesting: A Randomized Clinical Study. Photomed Laser Surg. 2016;34(6):263–71. https://doi.org/10.1089/pho.2016.4094. Erratum in: Photomed Laser Surg. 2018;36(1):58. https://doi.org/10.1089/pho.2016.4094.correx.
Willeit P, Tschiderer L, Allara E, Reuber K, Seekircher L, Gao L, ET AL. Carotid intima-media thickness progression as surrogate marker for cardiovascular risk: meta-analysis of 119 clinical trials involving 100 667 patients. Circulation. 2020 Aug 18;142[7]:621–642. https://doi.org/10.1161/CIRCULATIONAHA.120.046361. Epub 2020 Jun 17. PMID: 32546049; PMCID: PMC7115957.Yatsuya H. Risk Prediction, Encyclopedia of Cardiovascular Research and Medicine, Elsevier, 2018, Pages 315–318, ISBN 9780128051542.
Yoneda M, Uchiyama T, Kato S, Endo H, Fujita K, Yoneda K, Mawatari H, Iida H, Takahashi H, Kirikoshi H, Inamori M, Nozaki Y, Kobayashi N, Kubota K, Saito S, Maeyama S, Sagara M, Aburatani H, Kodama T, Nakajima A (2008) Plasma pentraxin 3 is a novel marker for nonalcoholic steatohepatitis (NASH). BMC Gastroenterol 14(8):53. https://doi.org/10.1186/1471-230X-8-53.PMID:19014569;PMCID:PMC2621235
Maleki I, Rastgar A, Hosseini V, Taghvaei T, Rafiei A, Barzin M, et al. High sensitive CRP and pentraxine 3 as noninvasive biomarkers of nonalcoholic fatty liver disease. Eur Rev Med Pharmacol Sci. 2014;18[11]:1583–90. PMID: 24943967.Choi B, Chung EJ. Pentraxine 3 [PTX3] as a biomarker of liver disease. In: Preedy VR [ed] Biomarkers in liver disease: methods, discoveries and applications. Biomedical and Life Sciences 2016, pp 1–20.
Ozturk K, Kurt O, Dogan T, Ozen A, Demirci H, Yesildal F, Kantarcioglu M, Turker T, Guler AK, Karslioglu Y, Altun B, Uygun A, Bagci S (2016) Pentraxin 3 is a predictor for fibrosis and arterial stiffness in patients with nonalcoholic fatty liver disease. Gastroenterol Res Pract. 2016:1417962. https://doi.org/10.1155/2016/1417962. (Epub 2016 Feb 22. PMID: 26997950; PMCID: PMC4779836)
Sookoian S, Gemma C, FernándezGianotti T, Burgueño A, Alvarez A, González CD et al (2007) Effects of rotating shift work on biomarkers of metabolic syndrome and inflammation. J Intern Med 261(3):285–292. https://doi.org/10.1111/j.1365-2796.2007.01766.x. (PMID: 17305651)
Yusuf S, Hawken S, Ounpuu S, Bautista L, Franzosi MG, Commerford P, Lang CC et al (2005) Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet 366(9497):1640–1649. https://doi.org/10.1016/S0140-6736[05]67663-5. (PMID: 16271645)
Ye X, Wang Z, Lei W, Shen M, Tang J, Xu X, Yang Y, Zhang H (2024) Pentraxin 3: a promising therapeutic target for cardiovascular diseases. Ageing Res Rev 93:102163. https://doi.org/10.1016/j.arr.2023.102163. (Epub 2023 Dec 11 PMID: 38092307)
Dubin R, Li Y, Ix JH, Shlipak MG, Whooley M, Peralta CA (2012) Associations of pentraxin-3 with cardiovascular events, incident heart failure, and mortality among persons with coronary heart disease: data from the Heart and Soul Study. Am Heart J 163(2):274–279. https://doi.org/10.1016/j.ahj.2011.11.007.PMID:22305847;PMCID:PMC3273726
Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, ET AL. National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003 May 21;289[19]:2560–72. https://doi.org/10.1001/jama.289.19.2560. Epub 2003 May 14. Erratum in: JAMA. 2003 Jul 9;290[2]:197. PMID: 12748199.
Bugianesi E, Gastaldelli A, Vanni E, Gambino R, Cassader M, Baldi S, Ponti V, Pagano G, Ferrannini E, Rizzetto M (2005) Insulin resistance in non-diabetic patients with non-alcoholic fatty liver disease: sites and mechanisms. Diabetologia. 48:634–42
Ishizaka N, Matsuzaki G, Saito K, Noiri E, Mori I, Nagai R (2006) Expression and localization of PDGF-B, PDGF-D, and PDGF receptor in the kidney of angiotensin II-infused rat. Lab Invest 86(12):1285–1292. https://doi.org/10.1038/labinvest.3700486. (Epub 2006 Oct 16 PMID: 17043664)
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
BH, team leader and supervision and conceptualization; EM, formal analysis, writing original draft, editing and publication; MM, data curation, formal analysis; GS, methodology; SM, methodology; TM, methodology; ST, data curation, formal analysis.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of the National Liver Institute, Menoufia University (NLI 00003413). Written informed consent was obtained from all participants.
Consent for publication
Written informed consent was obtained from all participants.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Badran, H., Elsabaawy, M., Magdy, M. et al. The utility of pentraxin 3 and platelet-derived growth factor receptor beta as non-invasive biomarkers for prediction of cardiovascular risk in MAFLD patients. Egypt J Intern Med 36, 86 (2024). https://doi.org/10.1186/s43162-024-00353-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43162-024-00353-1