Abstract
Clostridium perfringens, capable of causing intestinal infections in both animals and humans, represents a significant public health concern. This study aimed to assess the occurrence of the beta2 toxin-coding gene cpb2 in C. perfringens from various host species and to explore the genetic contexts of this gene. The results showed an enrichment of cpb2 in pig-derived C. perfringens. A comparative analysis of the detection rates of cpb2 and pCP13-like plasmids revealed that the cpb2 gene itself, rather than the pCP13-like plasmids, caused the enrichment. Sequence comparison of cpb2-positive pCP13-like plasmids showed that cpb2 was located on the cpb2-hp-transcriptional regulator (PadR family) segment. Despite the diverse plasmid structures of pCP13-like plasmids, the cpb2-hp-transcriptional regulator (PadR family) segment was consistently observed in all cpb2-positive C. perfringens strains, suggesting the potential transmission of the cpb2 gene on this specific genetic segment. Additionally, phylogenetic analysis of the C. perfringens strains harboring pCP13-like plasmids, as well as 31 pCP13-like plasmids, indicated that cpb2 did not affect the evolutionary relationship of either pCP13-like plasmids or C. perfringens. Genetic markers, particularly those located on mobile genetic elements (MGEs), that can help bacteria survive in external environments are more readily enriched in the population. The high prevalence of cpb2 in pig-derived strains indicated that it might confer a selective advantage, enhancing the survival and persistence of C. perfringens in the pig intestine. In conclusion, our study elucidated the genetic context, host tropism and potential biological functions of cpb2, which can provide references for further research.
Similar content being viewed by others
Introduction
Clostridium perfringens is a Gram-positive, rod-shaped, spore-forming bacterium that widely exists in natural environments and the intestinal tracts of both humans and animals [1]. C. perfringens strains can secrete more than 20 protein toxins or enzymes and are categorized into seven toxinotypes based on the combination of six major toxins (α-toxin, β-toxin, ε-toxin, ι-toxin, C. perfringens enterotoxin, and necrotic enteritis B-like toxin) [2].
Beta2 toxin (CPB2), with a molecular weight (MW) of approximately 28 kDa, was initially identified in a C. perfringens strain isolated from a piglet that died of necrotizing enterocolitis [3]. Subsequently, several studies have reported its presence in C. perfringens strains isolated from cases of enteritis and enterotoxaemia in both humans and animals [4,5,6,7]. Previous studies have indicated that CPB2 can be encoded by both cpb2 genes and atypical cpb2 (aty.cpb2) genes [8, 9], whereas the differences between the encoding proteins of these two genetic variants have not been elucidated. The aty.cpb2 genes share approximately 60–80% sequence identity with cpb2 genes but 99% identity with other aty.cpb2 genes. However, a high number of the aty.cpb2 genes have a frameshift mutation that prevented expression [9]. Therefore, this study focused on cpb2-positive C. perfringens strains.
The cpb2 gene was initially identified on plasmid pCP13 (AP003515.1). In contrast to the extensively studied pCW3-like plasmids, research on pCP13-like plasmids is relatively limited. It is currently known that pCP13-like plasmids are a conjugative plasmid family with a size ranging from ~ 36 kb to ~ 58 kb. In addition, they can carry cpb2 and the recently identified enterotoxin coding gene becA/B [8, 10, 11].
Epidemiological surveys and pathogenicity tests have indicated the potential involvement of CPB2 in enteric diseases and diarrhea in animals [12, 13]. Moreover, a recent study suggests that the primary effect of CPB2 is the formation of cation-selective channels [14]. High-throughput sequencing technology has been widely applied in epidemiological studies to trace and precisely control pathogenic agents. Nevertheless, there has been a lack of comprehensive research on the genetic characteristics of cpb2-positive C. perfringens strains and cpb2 genes. This study investigated the genomic features of cpb2-positive C. perfringens strains and explored the potential biological functions of cpb2 genes from both genomic and host-adapted perspectives, which can enhance our understanding of cpb2 gene and provide a reference for further research.
Results
Prevalence of toxin genes and pCP13-like plasmids among the C. perfringens genomes
The detection of toxin genes in the 763 C. perfringens genomes revealed the presence of plc in all samples, followed by cloSI (755/763, 98.95%), nanH (754/763, 98.82%), colA (733/763, 96.06%), nagH (682/763, 89.38%), nagJ (678/763, 88.86%), nanJ (675/763, 88.47%), nagI (674/763, 88.34%), nanI (657/763, 86.11%), pfoA (624/763, 81.78%), nagK (621/763, 81.39%), nagL (427/763, 55.96%), cpe (127/763, 16.64%), cpb2 (65/763, 8.52%), etx (61/763, 7.99%), netB (44/763, 5.77%), tpeL (36/763, 4.72%), cpb (28/763, 3.67%), becA/becB (9/763, 1.18%), and iap/ibp (8/763, 1.05%). Additionally, pCP13-like plasmids were identified in 30.3% (231/763) of the C. perfringens genomes.
cpb2 is associated with host species of C. perfringens
In order to eliminate potential bias introduced by the cpb2-positive strains in this study, we utilized genomic data from GenBank to compare the prevalence of cpb2 in C. perfringens strains across different hosts. The 757 C. perfringens genomes available in GenBank were classified into seven groups: human-derived strains (n = 216), chicken-derived strains (n = 108), sheep/goat-derived strains (n = 75), bovine-derived strains (n = 67), pig-derived strains (n = 65), strains of unknown origin (n = 46), and others (n = 180, including C. perfringens isolated from foods, environments, and other animals such as camels). The specific sources of the strains are listed in Table S1.
Among the 59 cpb2-positive C. perfringens strains available in GenBank, 25 strains were isolated from pigs, followed by humans (n = 5), chickens (n = 3), bovines (n = 3), and sheep/goats (n = 0).The remaining 23 cpb2-positive strains belonged to groups unknown (n = 9) and others (n = 14). Comparative analysis indicated that the detection rate of cpb2 was significantly higher (P < 0.01) in strains from pigs compared to those from humans, sheep/goats, bovines, and chickens (Table 1). This indicated that cpb2 or the pCP13-like plasmids capable of carrying cpb2 might affect the distribution of C. perfringens in different animals.
To ascertain the specific factor contributing to the high prevalence of cpb2 in pig-derived strains, we further investigated the prevalence of cpb2-negative pCP13-like plasmids from different sources. The results demonstrated significantly higher detection rates of cpb2-negative pCP13-like plasmids in C. perfringens from bovines (P = 6.97 × 10−6 , χ2 test) and humans (P = 0.01694, χ2 test) rather than pigs (Fig. 1A). These results indicated that the cpb2 gene but not the pCP13-like plasmids attributed to the enrichment of cpb2 in pig-derived C. perfringens.
Distribution of toxin genes among the C. perfringens strains from different sources. A Detection rates of cpb2-negative pCP13-like plasmids in different sources. B Box-plot of the toxin genes counts. C Volcano plot of the non-typing toxin genes between C. perfringens strains from pigs and bovines, D pigs and sheep/goats, E pigs and chickens, and F pigs and humans. The X-axis represents the difference in gene prevalence (in pigs-in other sources). The Y-axis represents the transformed P value (− Log10 P value). **: P < 0.01, ***: P < 0.001, NS.: no significance
Although more cpb2 genes were detected in the pig-derived strains, the total number of toxin genes was significantly lower in the pig-derived strains compared to those from sheep/goats, chickens, and bovines. (Fig. 1B). Therefore, we further investigated the prevalence of 11 non-typing toxin genes, including cloSI, colA, pfoA, nagH, nagI, nagJ, nagK, nagL, nanH, nanI, nanJ, in C. perfringens from different sources. The results indicated that the frequencies of nanI, nagK, and colA were significantly lower in pig-derived strains compared to those from sheep/goats, bovines, chickens, and humans (Fig. 1C–F). Additionally, the prevalence of pfoA was significantly lower in human-derived strains (Fig. 1F and Fig. S1). To eliminate any potential biases arising from researchers' preferences in strain selection and sequencing, the distribution of typing toxin genes was not examined in this study.
cpb2 doesn't affect the evolution relationship of C. perfringens
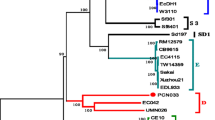
A total of 228 C. perfringens strains carrying pCP13-like plasmids, including 59 cpb2-positive and 169 cpb2-negative, passed the quality assessment of CheckM. A maximum likelihood tree was constructed based on 103,089 SNP sites across these 228 strains. The C. perfringens strains were divided into four clusters within the tree (Fig. 2). Approximately two-thirds of the cpb2-positive strains were found in Clusters I and II, while the remaining one-third were dispersed throughout the tree. Notably, the cpb2-positive strains in Clusters I and II typically originated from the same country or animal source, suggesting that the phylogenetic relationships among C. perfringens strains are more influenced by their isolation sources than the presence of the cpb2 gene. The cluster heatmap of ANIs also revealed the higher genomic similarity of C. perfringens strains from the same source (Fig. S2). Interestingly, several cpe-positive strains from humans and animals in the UK, the Netherlands, and Germany clustered together in Cluster III, indicating the potential transmission of cpe-positive C. perfringens strains among these countries.
Maximum likelihood tree of the 228 C. perfringens strains harboring pCP13-like plasmids. The toxin genes are represented by sky blue squares, plc, cpb, etx, cpe, netB, iap/ibp, cloSI, colA, cpb2, pfoA, nagH, nagI, nagJ, nagK, nagL, nanH, nanI, and nanJ in order from the inside to outside. Source and country of the C. perfringens strains were marked by squares with different colors
cpb2 locates on a conserved segment on pCP13-like plasmids
Six cpb2-positive pCP13-like plasmids were identified from the C. perfringens strains in this study: pscCP-7, pscCP-8, pscCP-25, pscCP-26, pscCP-28, and pscCP-97 (Table S2). Sequence comparisons together with plasmid pCP13 (AP003515.1) revealed that the Pcp loci were conserved across these plasmids (Fig. 3). The replication initiator gene of pCP13 was identified in pscCP-7, pscCP-8, pscCP-26, pscCP-28, and pscCP-97, but not in pscCP-25. It was observed that the cpb2 genes on these plasmids all located on cpb2-hp-transcriptional regulator (PadR family) segment. In addition, the resolvase coding gene resP and one hypothetical protein-coding gene (designated as tcb) were detected upstream of the cpb2 segments. Subsequently, we conducted gene detections of resP, tcb, and the cpb2 segment (cpb2, hp, and transcriptional regulator (PadR family)) among the 59 cpb2-positive C. perfringens strains. The results revealed that the cpb2 segment was detected in all cpb2-positive C. perfringens strains, whereas resP and tcb were detected from 49 and 32 of them, respectively. In addition, resP and tcb were always detected upstream of the cpb2 segment, indicating that they coexisted with cpb2 segment on the pCP13-like plasmids. The phylogenetic analysis of the 31 pCP13-like plasmids revealed that these plasmids with similar structural features clustered together in the evolutionary tree (Fig. S3), while the presence of cpb2 was not strongly correlated with the evolutionary relationship of the plasmids, suggesting that cpb2/cpb2-hp-transcriptional regulator (PadR family) was more likely to be a component of inter-plasmid transfer rather than a stabilizing component that had evolved over time.
Sequence comparison of the cpb2-positive plasmids in this study together with pCP13. Arrows indicate the directions of transcription of the genes, and shared regions are denoted by shadings. cpb2 genes, replication initiator coding genes, the Pcp locus coding genes, and other hypothetical proteins were marked with red, orange, green, and blue boxes, respectively
Discussion
C. perfringens is known for causing foodborne illnesses in humans and several gastrointestinal infections in animals [8, 15]. The pathogenicity of C. perfringens strains is primarily determined by the toxins they produce. It has been documented that C. perfringens can produce over 20 different protein toxins or enzymes. Among them, the biological characteristics and host pathogenicity of the six typing toxins have been extensively studied. Beta2 toxin is a pore-forming cytolytic toxin with less than 15% sequence homology to β-toxin [16]. Epidemiological studies have linked β2-toxin to intestinal diseases, such as necrotizing enteritis (NE) in piglets and enterocolitis in foals. Additionally, it has been suggested to be an accessory toxin in C. perfringens-associated non-foodborne diarrhea [1]. Although the structure of cpb2-positive plasmid pCP13 has been characterized to some extent [10], the genomic context of the cpb2 gene remains poorly understood, and there are no studies focusing on its host tropism or potential biological functions.
Epidemiological studies have indicated that a considerable proportion of C. perfringens strains obtained from pigs are positive for cpb2 [17, 18]. In a recent study [19], we detected toxin genes among animal-derived C. perfringens in China and observed that the majority of cpb2 genes were present in pig-derived C. perfringens strains. Here, we screened the distribution of toxin genes among 757 C. perfringens genomes available in GenBank and observed that there was an enrichment of cpb2 in pig-derived C. perfringens. Phylogenetic analysis of 228 C. perfringens strains harboring pCP13-like plasmids and 31 pCP13-like plasmids indicated that cpb2 was more likely to be a component of inter-plasmid transfer rather than a stabilizing component that has evolved over time. Additionally, the cpb2 genes were consistently located on the cpb2-hp-transcriptional regulator (PadR family) segment in all cpb2-positive C. perfringens strains, suggesting the potential horizontal transfer of cpb2 via this segment. Comparison of the detection rates of cpb2 and cpb2-negative pCP13-like plasmids across different sources indicated that cpb2 (or cpb2-hp-transcriptional regulator) but not pCP13-like plasmids led to the enrichment of cpb2 in pig-derived C. perfringens.
The presence or absence of genetic markers is often the result of bidirectional selection between bacterial strains and their hosts. Genetic markers that enhance bacterial resistance to external environments, without causing disease or lethality in hosts, are more likely to be enriched under selective pressure. The prevalence of mcr-1 during periods of heavy antibiotic use, followed by its decline after the colistin ban, supports this concept [20]. A recent study indicates that infant-associated pfoA + strains caused significantly more cellular damage to Caco-2 cells than pfoA − strains [21]. The higher pathogenicity of pfoA + strains and the lower detection rate of pfoA in human-derived strains was also consistent with the above concept. Although CPB2 can form cation-selective channels in cell membrane, only a prolonged incubation with CPB2 to 22 h induced significant propidium iodide (PI) entry into cells [14]. Additionally, an in vitro experiment has indicated that CPB2 does not contribute to cell cytotoxicity caused by human and porcine cpb2-harboring C. perfringens [22]. The higher prevalence of cpb2 among pig-derived strains suggests that the primary biological function of CPB2 might be promoting the survival of C. perfringens in pig intestines. In conclusion, this study highlighted the genetic context, host tropism, and potential biological functions of cpb2, which could provide references for further research.
Material and methods
Genome statistics
Six cpb2-positive C. perfringens strains (designated as scCP-7, scCP-8, scCP-25, scCP-26, scCP-28, scCP-97) isolated from pigs in Sichuan province were included in this study. C. perfringens isolation and cpb2 gene identification were performed in accordance with the methodology mentioned in our previous study [23]. Genomic DNA of the isolates was extracted using TIANamp Bacteria DNA Kit (Tiangen, Beijing, China) and subjected to whole-genome sequencing on the Illumina platform and Nanopore platform. Draft genomes were assembled using the hybrid mode of Unicycler 0.5.0 [24]. Genomes of 757 C. perfringens strains (as of November 10, 2023, genomic size > 2,700 KB, and plc positive) available on GenBank were analyzed together with the six C. perfringens strains in this study. Information on all C. perfringens strains is presented in Table S1.
Screening for genetic markers
Toxin genes of the C. perfringens strains were detected using VFDB (http://www.mgc.ac.cn/VFs/) and standalone BLASTn analyses. The conjugative locus of pCP13-like plasmids (Pcp locus) was identified by local BLASTn assays, and Pcp locus coding genes (pcpA-J) (AP003515.1) were used as reference sequences. Genomes positive for Pcp locus coding-genes were considered to contain pCP13-like plasmids.
Phylogenetic analysis
The genome quality of the C. perfringens strains carrying pCP13-like plasmids was assessed by checkM 1.0.12 [25]. Genomes with completeness > 95% and contamination < 5% were selected for further phylogenetic analysis. Average nucleotide identity (ANI) of the strains was identified by FastANI 1.34 (https://github.com/ParBLiSS/FastANI). Core genome single nucleotide polymorphisms (cgSNPs) among the C. perfringens strains were called using Snippy v4.6.0 (https://github.com/tseemann/snippy). A maximum likelihood tree was constructed based on the cgSNPs using FastTree 2.1.11 [26] and visualized using the iTOL online tool (https://itol.embl.de/). C. perfringens strains 13 (SAMD00061119) was used as the reference strain in the phylogenetic analysis. Grouping of the C. perfringens strains in the maximum likelihood tree was performed using rhierBAP [27].
Sequence comparison of the cpb2-positive pCP13-like plasmids
To gain insight into the genetic background of cpb2, we performed sequence comparison on the six cpb2-positive pCP13-like plasmids in this study together with plasmid pCP13. EasyFig 2.1 was used for the comparison and visualization of cpb2-positive pCP13-like plasmids [28]. Then, a set of 31 pCP13-like plasmids (Table S2), comprising 16 cpb2-positive plasmids and 15 cpb2-negative plasmids, available in GenBank were selected for sequence alignment and phylogenetic analysis. Sequence alignment of the plasmids was performed using MAFFT 7.520 [29], followed by phylogenetic tree construction using IQtree 2.2.5 [30]. The most appropriate substitution model was determined by the ModelFinder algorithm of IQTree.
Statistical analysis
Statistical analyses were performed using R version 4.3.2. Categorical data were analyzed using χ2 or Fisher’s exact test. Continuous data with normal and non-normal distributions were analyzed using the t-test and Mann–Whitney U test or Wilcoxon test, respectively.
Availability of data and materials
All genome assemblies of the C. perfringens strains were deposited in GenBank under BioProject accession number PRJNA1044929.
References
Kiu R, Hall LJ. An update on the human and animal enteric pathogen Clostridium perfringens. Emerg Microbes Infect. 2018;7(1):141.
Rood JI, Adams V, Lacey J, Lyras D, McClane BA, Melville SB, et al. Expansion of the Clostridium perfringens toxin-based typing scheme. Anaerobe. 2018;53:5–10.
Gibert M, Jolivet-Reynaud C, Popoff MR. Beta2 toxin, a novel toxin produced by Clostridium perfringens. Gene. 1997;203(1):65–73.
Manteca C, Daube G, Jauniaux T, Linden A, Pirson V, Detilleux J, et al. A role for the Clostridium perfringens beta2 toxin in bovine enterotoxaemia? Vet Microbiol. 2002;86(3):191–202.
Waters M, Savoie A, Garmory HS, Bueschel D, Popoff MR, Songer JG, et al. Genotyping and phenotyping of beta2-toxigenic Clostridium perfringens fecal isolates associated with gastrointestinal diseases in piglets. J Clin Microbiol. 2003;41(8):3584–91.
Waters M, Raju D, Garmory HS, Popoff MR, Sarker MR. Regulated expression of the beta2-toxin gene (cpb2) in Clostridium perfringens type A isolates from horses with gastrointestinal diseases. J Clin Microbiol. 2005;43(8):4002–9.
van Asten AJ, Nikolaou GN, Grone A. The occurrence of cpb2-toxigenic Clostridium perfringens and the possible role of the beta2-toxin in enteric disease of domestic animals, wild animals and humans. Vet J. 2010;183(2):135–40.
Gohari IM, Navarro MA, Li J, Shrestha A, Uzal F, McClane BA. Pathogenicity and virulence of Clostridium perfringens. Virulence. 2021;12(1):723–53.
Jost BH, Billington SJ, Trinh HT, Bueschel DM, Songer JG. Atypical cpb2 genes, encoding beta2-toxin in Clostridium perfringens isolates of nonporcine origin. Infect Immun. 2005;73(1):652–6.
Watts TD, Vidor CJ, Awad MM, Lyras D, Rood JI, Adams V. pCP13, a representative of a new family of conjugative toxin plasmids in Clostridium perfringens. Plasmid. 2019;102:37–45.
Ueda K, Kawahara K, Kimoto N, Yamaguchi Y, Yamada K, Oki H, et al. Analysis of the complete genome sequences of Clostridium perfringens strains harbouring the binary enterotoxin BEC gene and comparative genomics of pCP13-like family plasmids. BMC Genomics. 2022;23(1):226.
Luo R, Yang Q, Huang X, Yan Z, Gao X, Wang W, et al. Clostridium perfringens beta2 toxin induced in vitro oxidative damage and its toxic assessment in porcine small intestinal epithelial cell lines. Gene. 2020;759:144999.
Fisher DJ, Miyamoto K, Harrison B, Akimoto S, Sarker MR, McClane BA. Association of beta2 toxin production with Clostridium perfringens type A human gastrointestinal disease isolates carrying a plasmid enterotoxin gene. Mol Microbiol. 2005;56(3):747–62.
Benz R, Piselli C, Hoxha C, Koy C, Glocker MO, Popoff MR. Clostridium perfringens beta2 toxin forms highly cation-selective channels in lipid bilayers. Eur Biophys J. 2022;51(1):15–27.
Mellou K, Kyritsi M, Chrysostomou A, Sideroglou T, Georgakopoulou T, Hadjichristodoulou C. Clostridium perfringens foodborne outbreak during an athletic event in Northern Greece, June 2019. Int J Environ Res Public Health 2019;16(20):3967–71.
Hunter SE, Brown JE, Oyston PC, Sakurai J, Titball RW. Molecular genetic analysis of beta-toxin of Clostridium perfringens reveals sequence homology with alpha-toxin, gamma-toxin, and leukocidin of Staphylococcus aureus. Infect Immun. 1993;61(9):3958–65.
Li J, Zhou Y, Yang D, Zhang S, Sun Z, Wang Y, et al. Prevalence and antimicrobial susceptibility of Clostridium perfringens in chickens and pigs from Beijing and Shanxi. China Vet Microbiol. 2020;252:108932.
Chan G, Farzan A, Soltes G, Nicholson VM, Pei Y, Friendship R, et al. The epidemiology of Clostridium perfringens type A on Ontario swine farms, with special reference to cpb2-positive isolates. BMC Vet Res. 2012;8:156.
Wu K, Li Z, Fang M, Yuan Y, Fox EM, Liu Y, et al. Genome characteristics of the optrA-positive Clostridium perfringens strain QHY-2 carrying a novel plasmid type. mSystems. 2023;8(4):e0053523.
Wang Y, Xu C, Zhang R, Chen Y, Shen Y, Hu F, et al. Changes in colistin resistance and mcr-1 abundance in Escherichia coli of animal and human origins following the ban of colistin-positive additives in China: an epidemiological comparative study. Lancet Infect Dis. 2020;20(10):1161–71.
Kiu R, Shaw AG, Sim K, Acuna-Gonzalez A, Price CA, Bedwell H, et al. Particular genomic and virulence traits associated with preterm infant-derived toxigenic Clostridium perfringens strains. Nat Microbiol. 2023;8(6):1160–75.
Allaart JG, van Asten AJ, Vernooij JC, Grone A. Beta2 toxin is not involved in in vitro cell cytotoxicity caused by human and porcine cpb2-harbouring Clostridium perfringens. Vet Microbiol. 2014;171(1–2):132–8.
Wu K, Feng H, Ma J, Wang B, Feng J, Zhang H, et al. Prevalence, toxin-typing and antimicrobial susceptibility of Clostridium perfringens in sheep with different feeding modes from Gansu and Qinghai provinces. China Anaerobe. 2022;73:102516.
Wick RR, Judd LM, Gorrie CL, Holt KE. Unicycler: Resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput Biol. 2017;13(6):e1005595.
Parks DH, Imelfort M, Skennerton CT, Hugenholtz P, Tyson GW. CheckM: assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res. 2015;25(7):1043–55.
Price MN, Dehal PS, Arkin AP. FastTree: computing large minimum evolution trees with profiles instead of a distance matrix. Mol Biol Evol. 2009;26(7):1641–50.
Tonkin-Hill G, Lees JA, Bentley SD, Frost SDW, Corander J. RhierBAPS: An R implementation of the population clustering algorithm hierBAPS. Wellcome Open Res. 2018;3:93.
Sullivan MJ, Petty NK, Beatson SA. Easyfig: a genome comparison visualizer. Bioinformatics. 2011;27(7):1009–10.
Katoh K, Rozewicki J, Yamada KD. MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief Bioinform. 2019;20(4):1160–6.
Minh BQ, Schmidt HA, Chernomor O, Schrempf D, Woodhams MD, von Haeseler A, et al. IQ-TREE 2: New models and efficient methods for phylogenetic inference in the genomic era. Mol Biol Evol. 2020;37(5):1530–4.
Acknowledgements
Not applicable.
Funding
This work was supported by the Natural Science Basic Research Plan in Shaanxi Province of China (No. 2021-KW-41), the China Agriculture Research System of MOF and MARA (CARS-39) and Ganzi Prefecture Science and Technology Plan Project of 2022 (22kjjh0016).
Author information
Authors and Affiliations
Contributions
J.W. and Z.Y. conceived and supervised the study; J.W., D.Y. and K.W. designed the experiments and wrote the manuscript; K.W., Y.Y. and M.F. performed the experiments and analyzed the data; L.Z., Y.L. and X.T. helped with experiments; L.D., D.Y. and E.F. took part in the editing of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare no conflicts of interest regarding the publication of this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wu, K., Yuan, Y., Fang, M. et al. Genomic insights into cpb2-positive Clostridium perfringens and the potential biological function of cpb2 gene. One Health Adv. 2, 25 (2024). https://doi.org/10.1186/s44280-024-00058-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1186/s44280-024-00058-8