Abstract
Cephalexin (CFX), a β-lactam antibiotic which is identified for the treatment of different disease infections, has been reported as a micropollutant in wastewater released from sewage, hospital, or pharmaceutical industries. Chlorella pyrenoidosa-2378, a green microalgal strain, is recognized for its degradation ability against wastewater pollutants and the potential of the biomass it produces. In this present study, the cultivation process of Chlorella pyrenoidosa-2378 strain with CFX concentration of 50 mg/L, 100 mg/L, 150 mg/L, and 200 mg/L added to its culture medium labeled as test I, test II, test III, and test IV, respectively, is the process being observed for evaluation of its degradation ability and as sustainable approach for antibiotic degradation. chlorophyll a in test IV, chlorophyll b in test III, and carotenoid content in test I were observed to be higher in amount than control by 0.775 mg/L, .069 mg/L, and 0.356 mg/L, respectively. Using the HPLC method, the total removal efficiency was observed to be 79.09%, 86.26%, 89.62%, and 88.03% against 50, 100, 150, and 200 mg/L concentrations of CFX, respectively. The observations that C. pyrenoidosa-2378 remained stable while being used as an alternative bioremediation method, provide an alibi for its novelty and potentiality to be used commercially for biomass production and at industrial scale for degradation.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Cephalexin (CFX) scientifically named as 7-(D-a-Amino-a-phenylacetamido)-3-methyl-3-cephem-4-carboxylic acid monohydrate) (C16H17O4 N3S·H2O), a cephalosporin, is a combination of beta-lactam and a dihydrothiazide which is used against skin and skin structure, gastrointestinal and genitourinary tract infections [1,2,3]. This first-generation cephalosporin antibiotic, which is ranked among the top three antibiotics, globally has now been categorized as an emerging contaminant in water [4].
Current antibiotic market growth expects a CAGR of 5.25% with a $ 20.59 billion increase in the global production of antibiotics in 2021 (40.79 billion), at the end of the forecast period from 2021–2029. A 0.2–0.3% increase in cephalosporins sales was observed with a 10% rise in the monthly rate of COVID-19 cases between 2020 and 2022 [5]. Cephalosporin Market was valued at USD 15.47 Billion in 2023 and is expected to reach USD 19.75 Billion by the end of 2031 with a CAGR of 3.10% During the Forecast Period 2024–2031 [6]. This process of increase in production rate directly leads to a rise in pollutant concentration in wastewater effluents from household, municipal, and pharmaceutical factories [7]. Current existing pharmaceutical wastewater treatment strategies such as advanced oxidation, activated carbon, anaerobic digestion, and vacuum evaporation, are applied in combination as they only offer partial treatment and a sudden rise observed might not make them able to deliver proper treatment efficiency individually [8]. These strategies when combined and applied, contribute to extra cost and energy requirements [9]. Also contributing to another set of contaminants added to the list of emerging pollutants in the environment, the effluents when combined and mixed create another threat as the concentration of the antibiotic might change from ng/L to μg/L [10, 11]. Therefore, the treatment strategy applied should be sustainable and able to treat every new pollutant in wastewater along with the ability to deal with the unpredicted hike in antibiotic concentration [12].
Microalgae has been recognized as an effective and sustainable treatment strategy for many emerging and persistent environmental pollutants from any source, including the contaminants from pharmaceutical industry [12,13,14]. As the pollutants are fixed permanently in algal biomass, the toxic biomass produced can be processed into valuable bioactive compounds [15, 16]. Chlorella pyrenoidosa-2378, a microalgal strain has been reported for its effectiveness in wastewater treatment from different sources, and the potential its biomass possesses to produce biodiesel, biogas, bioelectricity, etc. [17,18,19]. This strain has a potential for degrading antibiotics such as Ciprofloxacin and Norfloxacin, with individual effectiveness in antibiotic removal achieved at 79% for Ciprofloxacin (initial concentration 0.029 µg/L) and 50% for Norfloxacin (initial concentration 0.032 µg/L) [20]. The main objective of this research study is to investigate the degradation ability, tolerance, and stability of Chlorella pyrenoidosa–2378, a fresh-water green microalgae, against the relatively high concentration of CFX it encounters during its cultivation process. Throughout the degradation process if the culture is stable, then the cultivation process will not be affected by the antibiotic present in the medium and the biomass will be stable enough for further processing into products.
Methods and methodology
Chemicals
Cephalexin (CFX) (CAS No.: 15686–71-2) (HPLC grade, > 99% purity) was purchased from Combi-Blocks, Inc, (USA). K2HPO4, NaNO3, Na2CO3, EDTANa2, Na2MoO4·2H2O, MgSO4·7H2O, CaCl2·2H2O, MnCl2·4H2O, ZnSO4·7H2O, CuSO4·5H2O, Co (NO3)2·6H2O, H3BO3, citric acid, and ferric ammonium citrate were purchased from Hi-Media Laboratories. Methanol and Acetonitrile were purchased from Advent Chembio Pvt. Ltd. in Mumbai, India, respectively. All the chemicals and materials used in the experiment were of HPLC and analytical grade respectively.
Algal strain and culture cultivation conditions
The microalgae strain, Chlorella pyrenoidosa-2378, was procured from the National Collection of Industrial Microorganisms (NCIM), CSIR-NCL, (Pune) India. The strain was cultured and subcultured in BG-11 Medium in 250 mL Erlenmeyer flasks to provide sufficient volume for experimental runs. With the macronutrient solution composition of NaNO3 1500 mg/L, K2HPO4 40 mg/L, MgSO4.7H2O 75 mg/L, CaCl2.2H2O 36 mg/L, citric acid 6 mg/L and micronutient composition of FeC6H5O7.NH4OH 6 mg/L, Na2-EDTA mg/L, MnCl2.4H2O 1.81 mg, ZnSO4.7H2O 0.222 mg, Na2MoO4.2H2O 0.39 mg/L, CuSO4.5H2O 0.08 mg/L, H3BO3 2.86 mg/L, BG-11 Medium was prepared. The macronutrient solution was sterilized in an autoclave at 15 psi, 121 °C for 15 min, while the micronutrient solution was sterilized using a 0.45-μm pore size syringe filter (Axiva Sichem Pvt. Ltd.) and a 20-ml plastic syringe. The medium was then inoculated with the above-prepared 1.5% C.pyrenoidosa suspension (Vinoculum/Vmedium). The inoculated medium flasks were incubated for 11 days, at 27 °C with constant shaking at 150 rpm, provided with illumination under a white, fluorescent lamp of light intensity of 30–35/mol photon m−2 s−1 and 16/8 h photoperiod [21]. The microalgal suspension (11 days old), to be used as an inoculum for further experiments, was first diluted with sterilized BG-11 Medium till the optical density (O.D.) of 1.0 was achieved at 680 nm. Absorbance values were observed using a UV/Visible spectrophotometer (Evolution 201, Thermo Scientific, USA).
Experimental design for growth inhibition assay
Further, for the assessment of antibiotic degradation ability, and evaluating the stability and tolerance of the procured strain, sterilized 150 mL BG-11 Medium was prepared in fifteen 250 mL Erlenmeyer flasks. The medium was inoculated with the above prepared 1.5% C.pyrenoidosa suspension (Vinoculum/Vmedium). The stock solution of CFX was prepared by dissolving the antibiotic in ultra-pure distilled water. For performing growth inhibition assay, the algal culture in inoculated flasks was exposed to different CFX concentrations of 50 mg/L, 100 mg/L, 150 mg/L, and 200 mg/L labeled as test I, test II, test III, and test IV, respectively. The flasks were incubated for 11 days under the conditions mentioned in the “Algal strain and culture cultivation conditions” section. The experiment was performed in triplicates, for evaluation of the complete removal efficiency of CFX by C. pyrenoidosa by comparing the analytical results in test samples I, II, III, and IV (50, 100, 150, 200 mg) with control (C) (inoculated medium without antibiotic). For determining the abiotic removal of CFX, the same concentration of CFX was added to the culture flasks in the absence of microalgae.
Determination of biomass concentration and total chlorophyll and carotenoid content for Chlorella pyrenoidosa-2378
The biomass concentration was determined by observing the optical density of C. pyrenoidosa at 680 nm (OD680) using the UV/Visible spectrophotometer [22]. The extraction of the photosynthetic pigments, chlorophyll a, chlorophyll b, and carotenoid, was performed by carrying out centrifugation of the culture, at 4500 rpm (15 min) [23]. The pellet was suspended in 10 mL of 90% methanol and incubated for 10 min. This was performed twice before the absorbance of the supernatant obtained was measured at 665 nm, 652 nm, and 470 nm, and the chlorophyll a (Chla), chlorophyll b (Chlb), and carotenoids were estimated using the formulas mentioned below [24]:
Morphological characterization
Light microscopy
Microalgae strains were investigated under a light microscope using a bright field and differential contrast on rehydrated biofilms as well as liquid cultures. Morphological modifications were photographed at × 10–40 magnifications before and after the test using a (Radical RXLT-4 IT) microscope linked to a digital camera system.
Scanning electron microscopy
However, the more detailed morphology of the cells C. pyrenoidosa-2378 was studied using JSM 6490 Scanning Electron Microscope (SEM) [25]. The samples were prepared by centrifuging 100 ml of culture (control and test) at 4000 rpm for 10 min and rinsing them in 0.1 M phosphate buffer (pH 7.5). Following many rounds of washing with the same buffer, the samples were dehydrated in ethanol at various concentrations of 25%, 50%, 75%, 95%, and 100% (v/v). A scanning electron microscope (JSM 6490) was used to conduct the treatment observations at various magnifications operating at 10 kV (as observed in Fig. 1).
Microscopic image of Chlorella pyrenoidosa pre- and post-treatment: A SEM image of control at × 1000 magnification. B SEM image of the test sample at 200 mg/L concentration at × 1000 magnification. C Light microscopy of the control sample at 40 × . D Light microscopy of the test sample of 200 × at × 40 magnification respectively
HPLC analysis
Two milliliters of culture were withdrawn from each flask on days 0, 2nd, 4th, 7th, and 11th. The withdrawn samples were centrifuged at the speed of 10,000 rpm for 10 min. The residual concentration of CFX in aqueous medium was measured by filtering the sample’s supernatant through a 0.20-µm membrane filter and further carrying out analysis by performing HPLC.
CFX concentrations were determined by using HPLC (Agilent Technologies-1200) with an Auto-sampler and DAD. Five microliters of sample were injected into a C18 column (250 × 4.6 mm, 5 µm) which was kept at ambient temperature. Water, methanol, and acetonitrile (60:20:20 v/v) were used as the mobile phase at a flow rate of 0.4 mL min1. The concentration of CFX was determined by measuring the column effluent at 254 nm.
Statistical analysis
The data were statistically analyzed using GraphPad Prism version 5.0 for Windows (USA), one-way analysis of variance, and the Tukey–Kramer multiple comparison tests. When p < 0.05, differences were considered significant.
Results and discussion
Effect of CFX on growth of Chlorella pyrenoidosa-2378
The cells were observed morphologically through light microscopy and SEM and were observed to be ruptured and swelled up due to absorption of CFX as compared to the control. The optical density was used as the first reference to evaluate the stability of the culture throughout the degradation process.
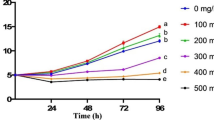
The current research aimed to examine the growth patterns of C. pyrenoidosa in cultures subjected to varying concentrations of CFX. Moreover, through the process of deducing the correlation between various concentrations of CFX and the absorbance observed at 680 nm, the relationship between the biomass of C. pyrenoidosa and the different concentrations of CFX was then evaluated (Fig. 2). The OD680 values increased steadily from day 0 to day 11 in control and test III. The initial decline in OD680 values by 0.012 and 0.015 were observed on day 2 in tests I and II, respectively. Although steady, the increase in OD680 values in test IV with every time period gap was observed to be minimal with only 0.02 difference from day 0 to day 2 and 0.07 from day 2 to day 4.
The observed growth patterns of C. pyrenoidosa in all experimental setups exhibited statistically significant differences (p > 0.05). These results suggest that higher concentrations of CFX (150 mg/L and 200 mg/L) had a significant inhibitory effect on the growth pattern of C. pyrenoidosa. Conversely, lower concentrations of CFX (50 mg/L) appeared to promote the growth of the microalgal culture. The findings of this study indicate the potential existence of hormesis, a phenomenon characterized by a biphasic response to dosage. Specifically, it was observed that a low dose of the substance under investigation had a positive impact on the growth of the organism, while a high dose had an inhibitory effect on growth [26]. This can be supported by the observation that freshwater microalgal strains are observed to be getting contaminated over time in the open cultivation process, but the introduction of antibiotics might keep the culture in check, keeping it away from any aerial contamination. The reported decline in growth can be attributed to the induction of oxidative stress in the chloroplasts because of antibiotic exposure, as reported by [27].
Effect of CFX on photosynthetic pigments of Chlorella pyrenoidosa-2378
The phenomenon of pigmentation system modification has been widely recognized as a defensive response mechanism in various stress-inducing scenarios, as documented by [28]. The pigments commonly found in microalgae, such as chlorophyll (including Chl-a, Chl-b, and carotenoids), are widely recognized as a hallmark of cellular adaptability. Chlorophyll, a vital pigment found in plants and other photosynthetic organisms, assumes a crucial role in the process of photosynthesis. In addition, it is notable that this phenomenon also assumes a significant function in the process of energy transfer, light energy conversion, and light harvesting. Additionally, the process of growth inhibition and the complex production of organic components are closely related to these phenomena [29]. According to research by [30] antibiotics have a deactivating impact on the production of photosynthetic pigments. The pigment content of Chlorella vulgaris dramatically reduced under clarithromycin stress, according to a study [31]. While it is widely observed that stress-induced conditions often lead to a decrease in chlorophyll content, there have been reports documenting an intriguing phenomenon of increased microalgae content when exposed to low concentrations of antibiotics, and conversely, a reduction in content at higher doses. The impact of CFX stress on the composition of chlorophyll a, b, and carotenoids exhibited similar patterns of alteration. The levels of chlorophyll a, chlorophyll b, and carotenoids. A steady increase in Chla values was observed from day 0 to day 11 in every sample (Fig. 3). A decline was only observed on day 11(5.500 mg) from day 7 (5.363 mg) in test II. Almost 80% of values observed among Chla values test I, II, II, and IV were higher than the value observed in control from day 2 to day 11. The highest recorded concentration of Chla was 5.740 mg in test III, followed by test IV, II, control, and test I. The highest increase in Chla content from day 0 to day 11, was observed in test III by 5.319 mg (day 0 0.421 mg to day 11 5.740 mg). Similarly, the Chlb values recorded were highest on day 11 (2.924 mg) in test III followed by the Chlb content in control (Fig. 4). Comparing the increase from day 0 to day 11, the highest increase in content by 2.66 mg was observed in test III (day 0 0.264; day 11 2.924). A steady increase in carotenoid content was observed in control and all the test samples (Fig. 5). A maximum increase of 1.455 mg from day 0 to day 11 was observed in test I (day 0 0.116; day 11 1.571 mg). The initial values of test II and test III were at closest with only a gap of 0.004 mg and 0.007 on day 2 when compared with the test I value and the gap reached 0.154 mg and 0.517 on day 11, respectively.
Removal of CFX by Chlorella pyrenoidosa-2378
The degradation of CFX was measured and analyzed through HPLC analysis (Figs. 6, 7, 8, and 9). The 11 days microalgal cultivation in presence of CFX at different concentration resulted in total degradation efficiency to be in between 79-90%. Fifty-three to 59% CFX was observed to be degraded during the time of first 2 days (Fig. 10).
During the time interval day 2–day 11, only about 21% of CFX of the sample was degraded in test I, while 30.12% of CFX was degraded in test II. In test III and test IV, approximately 35% of CFX was degraded during this period. Only an amount between 10-21% remained after the 11-day period degradation testing process ended. The residual concentration of CFX measured after degradation by C.pyrenoidosa on Days 2,4,7 and 11, analyzed using HPlC has been mentioned below in Table 1.
Discussion
The green microalgal strain, Chlorella Pyrenoidosa–2378, is not targeted by the antibiotic during the degradation process, yet its effect on this strain cannot be rendered null and void. The increase in OD680 values from day 0 to day 11, was observed in the order of C > I > II > III > IV. The increase in Chla content from day 0 was observed as IV > III > II > C > I. The increase in Chlb content was observed as III > C > II > I > IV. The increase in Carotenoid content was observed as I > IV > II > C > III. The OD680 values were affected directly as the values decreased with respect to the increase in CFX concentration. The test IV had maximum CFX concentration but still had a maximum increase in its Chla content, around 0.611 mg more than the control. Also, Chlb content was still observed to increase nearly the maximum in test III (2.66 mg/L), followed by control (2.591 mg/L). No direct linear relationship was observed between CFX concentration and pigment concentration in the samples. As the pigment content observed was even in higher quantity than the control, this provides an alibi that the presence of antibiotics is a supportive choice while aiming for higher pigment concentration in C. pyrenoidosa. This directly points towards the fact that this microalgal strain has the ability to deal with antibiotic toxicity and cause its degradation at an almost acceptable rate with 79–90% efficacy. The research conducted by Chen and coworkers highlights the significance of the heterotrophic capabilities and endogenous catabolic mechanisms demonstrated by microalgae [32]. C. vulgaris and C.pyrenoidosa are among the most studied microalgal cultures used for micropollutant degradation, including cephalosporins [33]. In a research study, non-living Chlorella lipid extracted was used to remove CFX (49.17 ± 0.20 mg/L concentration) showed a maximum efficient removal to be 71.19% with respect to control (82.77%) using 14.17 ± 5.52 of lipid extracted biomass [34]. The research study conducted on the degradation of CFX using a microalgae-bacteria consortium by da Silva Rodrigues and his coworkers reported degraded 96.54% of CFX, which seems to prove great results but may become a threat as the repetitive use of bacteria for degradation might turn it into a threat [35]. Nano-zero-valent iron from Nettle leaf extract can remove 1667 mg of CFX, but its preparation requires Nettle leaf extract, which makes the process less sustainable as the production might become a threat to biodiversity leading to its loss in the near future [36].
Conclusion
Different sustainable approaches have been recognized for removing CFX For removing CFX [36]. Comparing every single approach the most accepted approach should be the one with the least by-products that damage the environment without leading to additional pollution or threat and the second is less costly. Microalgal-based treatment should be considered efficient because (a) the by-products are not a threat, (b) Oxygen production, (c) considering air pollution, the combined process might even improve air levels when used in industrial areas (c) biomass can be used for biodiesel, bioelectricity, bioethanol, immune-supplement products, and protein production [37, 38]. The pathway followed by microalgae for degrading antibiotics or any other micropollutant is bioabsorption, bioaccumulation, and biodegradation [33]. The findings of this research indicate that C. pyrenoidosa may establish resistance against CFX toxicity and consequently, efficiently remove CFX from aqueous environments. As a result, C. pyrenoidosa might be a potential microalga for CFX-contaminated water remediation. Using this strain, which has been identified for bioremediation of different air, water, and soil pollutants, identifying its additional application in CFX degradation, turns this strain into a novel promising approach and an economically feasible method that could be used at a large scale. In the future, the potential of C. pyrenoidosa for the removal of other major contaminants can be investigated.
Availability of data and materials
No datasets were generated or analysed during the current study.
Abbreviations
- WHO:
-
World Health Organization
- HPLC:
-
High-performance liquid chromatography
- MSSA:
-
Methicillin-susceptible Staphylococcus aureus
- DDD:
-
Defined daily doses
- CAGR:
-
Compound annual growth rate
- COVID:
-
Coronavirus disease (COVID-19)
- CDC:
-
Centers for Disease Control and Prevention
- CFX:
-
Cephalexin
- SEM:
-
Scanning electron microscope
References
Kanan M, Atif S, Mohammed F, Balahmar Y, Adawi Y, AlSaleem R, Farhan A, Alghoribi M, Mohammed S, Alshanbari R, Fahad M. A systematic review on the clinical pharmacokinetics of cephalexin in healthy and diseased populations. Antibiotics. 2023;12(9):1402.
Okumura N, Hayakawa K, Yamamoto K, Yamada G, Mezaki K, Ohmagari N. Effectiveness of oral cephalexin in antibiotic-course completion for methicillin-susceptible Staphylococcus aureus-induced bacteremic vertebral osteomyelitis. BMC Infect Dis. 2023;23(1):307. https://doi.org/10.1186/s12879-023-08266-0.
Al-Gburi LJ, Al-Noor TH. Metal complexes of cephalexin and their biological activities: a review. https://doi.org/10.37591/JoPC.
Benarab N, Fangninou FF. The issues of antibiotics: cephalexin antibiotic as emerging environment contaminant. Int J Sci Res Publ. 2020;10(2):306–18.
Data Bridge Market Research. Global Antibiotic Production Market – Industry Trends and Forecast to 2029. 2022. https://www.databridgemarketresearch.com/reports/global-antibiotic-production-market. Accessed 27 April 2023.
Global Cephalosporin Market – Industry Trends and Forecast to 2031. DBMR Nucleus Solution. 2024. https://www.databridgemarketresearch.com/reports/global-cephalosporins-market.
Benarab N, Fangninou FF. The issues of antibiotics: cephalexin antibiotic as emerging environment contaminant. Int. J. Sci. Res. Publ. 2020;10(2):306–18. https://doi.org/10.29322/IJSRP.10.02.2020.p9843.
Omuferen LO, Maseko B, Olowoyo JO. Occurrence of antibiotics in wastewater from hospital and convectional wastewater treatment plants and their impact on the effluent receiving rivers: current knowledge between 2010 and 2019. Environ Monit Assess. 2022;194(4):306.
Rodríguez-Serin H, Gamez-Jara A, De La Cruz-Noriega M, Rojas-Flores S, Rodriguez-Yupanqui M, Gallozzo Cardenas M, Cruz-Monzon J. Literature review: evaluation of drug removal techniques in municipal and hospital wastewater. Int J Environ Res Public Health. 2022;19(20):13105.
Fakhri BMS, Ghassemi Barghi N, Moradnia Mehdikhanmahaleh M, Raeis Zadeh SM, Mousavi T, Rezaee R, Daghighi M, Abdollahi M. Pharmaceutical wastewater toxicity: an ignored threat to the public health. Sustainable Environment. 2024;10(1):2322821.
Adeleye AS, Xue J, Zhao Y, Taylor AA, Zenobio JE, Sun Y, Han Z, Salawu OA, Zhu Y. Abundance, fate, and effects of pharmaceuticals and personal care products in aquatic environments. J Hazard Mater. 2022;15(424):127284.
Rempel A, Gutkoski JP, Nazari MT, Biolchi GN, Biduski B, Treichel H, Colla LM. Microalgae growth with a high concentration of emerging pollutants and phytotoxicity evaluation of cultivation wastewater. Journal of Water Process Engineering. 2022;1(46):102616.
De Mendonca HV, Assemany P, Abreu M, Couto E, Maciel AM, Duarte RL, dos Santos MG, Reis A. Microalgae in a global world: new solutions for old problems? Renewable Energy. 2021;1(165):842–62.
Singh DV, Upadhyay AK, Singh R, Singh DP. Persistent organic pollutants: sources, impacts, and their remediation by microalgae. In: Environmental Biotechnology. Apple Academic Press; 2022. p. 57-86. https://doi.org/10.1201/9781003277279.
Amaro HM, Salgado EM, Nunes OC, Pires JC, Esteves AF. Microalgae systems-environmental agents for wastewater treatment and further potential biomass valorisation. J Environ Manage. 2023;1(337):117678.
Yu C, Pang H, Wang JH, Chi ZY, Zhang Q, Kong FT, Xu YP, Li SY, Che J. Occurrence of antibiotics in waters, removal by microalgae-based systems, and their toxicological effects: A review. Sci Total Environ. 2022;20(813):151891.
Kumari P, Varma AK, Shankar R, Thakur LS, Mondal P. Phycoremediation of wastewater by Chlorella pyrenoidosa and utilization of its biomass for biogas production. J Environ Chem Eng. 2021;9(1): 104974. https://doi.org/10.1016/j.jece.2020.104974.
Bhatt A, Arora P, Prajapati SK. Chlorella pyrenoidosa-mediated removal of pathogenic bacteria from municipal wastewater–Multivariate process optimization and application in the real sewage. J Environ Chem Eng. 2023;11(2):109494.
Hao TB, Balamurugan S, Zhang ZH, Liu SF, Wang X, Li DW, Yang WD, Li HY. Effective bioremediation of tobacco wastewater by microalgae at acidic pH for synergistic biomass and lipid accumulation. J Hazard Mater. 2022;15(426):127820.
Ricky R, Shanthakumar S. An investigation on removal of ciprofloxacin and norfloxacin by phycoremediation with an emphasis on acute toxicity and biochemical composition. Sci Rep. 2023;13(1):13911.
Gunawan TJ, Ikhwan Y, Restuhadi F, Pato U. Effect of light Intensity and photoperiod on growth of Chlorella pyrenoidosa and CO2 Biofixation. In: E3S Web of Conferences, vol. 31. EDP Sciences; 2018. p. 03003. https://doi.org/10.1051/e3sconf/20183103003.
Ho SH, Chen CY, Chang JS. Effect of light intensity and nitrogen starvation on CO2 fixation and lipid/carbohydrate production of an indigenous microalga Scenedesmus obliquus CNW-N. Biores Technol. 2012;1(113):244–52. https://doi.org/10.1016/j.biortech.2011.11.133.
Ji MK, Kabra AN, Choi J, Hwang JH, Kim JR, Abou-Shanab RA, Oh YK, Jeon BH. Biodegradation of bisphenol A by the freshwater microalgae Chlamydomonas mexicana and Chlorella vulgaris. Ecol Eng. 2014;1(73):260–9. https://doi.org/10.1016/j.ecoleng.2014.09.070.
Xiong JQ, Kurade MB, Abou-Shanab RA, Ji MK, Choi J, Kim JO, Jeon BH. Biodegradation of carbamazepine using freshwater microalgae Chlamydomonas mexicana and Scenedesmus obliquus and the determination of its metabolic fate. Biores Technol. 2016;1(205):183–90. https://doi.org/10.1016/j.biortech.2016.01.038.
Hossain N, Zaini J, Mahlia TM, Azad AK. Elemental, morphological and thermal analysis of mixed microalgae species from drain water. Renewable Energy. 2019;1(131):617–24. https://doi.org/10.1016/j.renene.2018.07.082.
Christou A, Michael C, Fatta-Kassinos D, Fotopoulos V. Can the pharmaceutically active compounds released in agroecosystems be considered as emerging plant stressors? Environ Int. 2018;1(114):360–4. https://doi.org/10.1016/j.envint.2018.03.003.
Li J, Zheng X, Liu K, Sun S, Li X. Effect of tetracycline on the growth and nutrient removal capacity of Chlamydomonas reinhardtii in simulated effluent from wastewater treatment plants. Biores Technol. 2016;1(218):1163–9. https://doi.org/10.1016/j.biortech.2016.07.080.
Zhang L, Pei H, Chen S, Jiang L, Hou Q, Yang Z, Yu Z. Salinity-induced cellular cross-talk in carbon partitioning reveals starch-to-lipid biosynthesis switching in low-starch freshwater algae. Biores Technol. 2018;1(250):449–56. https://doi.org/10.1016/j.biortech.2017.11.067.
Nie X, Wang X, Chen J, Zitko V, An T. Response of the freshwater alga Chlorella vulgaris to trichloroisocyanuric acid and ciprofloxacin. Environmental Toxicology and Chemistry: An International Journal. 2008;27(1):168–73. https://doi.org/10.1897/07-028.1.
Qian H, Li J, Pan X, Sun Z, Ye C, Jin G, Fu Z. Effects of streptomycin on growth of algae Chlorella vulgaris and Microcystis aeruginosa. Environ Toxicol. 2012;27(4):229–37. https://doi.org/10.1002/tox.20636.
Guo J, Peng J, Lei Y, Kanerva M, Li Q, Song J, Guo J, Sun H. Comparison of oxidative stress induced by clarithromycin in two freshwater microalgae Raphidocelis subcapitata and Chlorella vulgaris. Aquat Toxicol. 2020;1(219):105376. https://doi.org/10.1016/j.aquatox.2019.105376.
Chen J, Zheng F, Guo R. Algal feedback and removal efficiency in a sequencing batch reactor algae process (SBAR) to treat the antibiotic cefradine. PLoS ONE. 2015;10(7):e0133273. https://doi.org/10.1371/journal.pone.0133273.
Liu R, Li S, Tu Y, Hao X. Capabilities and mechanisms of microalgae on removing micropollutants from wastewater: a review. J Environ Manage. 2021;1(285):112149.
Angulo E, Bula L, Mercado I, Montaño A, Cubillán N. Bioremediation of Cephalexin with non-living Chlorella sp., biomass after lipid extraction. Bioresource technology. 2018;257:17–22.
da Silva Rodrigues DA, da Cunha CC, do Espirito Santo DR, de Barros AL, Pereira AR, de Queiroz Silva S, da Fonseca Santiago A, de Cássia Franco Afonso RJ. Removal of cephalexin and erythromycin antibiotics, and their resistance genes, by microalgae-bacteria consortium from wastewater treatment plant secondary effluents. Environ Sci Pollut Res Int. 2021;28:67822–32.
Fazlzadeh M, Ansarizadeh M, Leili M. Data of furfural adsorption on nano zero valent iron (NZVI) synthesized from Nettle extract. Data Brief. 2018;1(16):341–5.
Noman EA, Al-Gheethi A, Mohamed RM, Talip BA, Hossain MS, Altowayti WA, Ismail N. Sustainable approaches for removal of cephalexin antibiotic from non-clinical environments: a critical review. J Hazard Mater. 2021;5(417):126040.
Hom-Diaz A, Jaén-Gil A, Rodríguez-Mozaz S, Barceló D, Vicent T, Blánquez P. Insights into removal of antibiotics by selected microalgae (Chlamydomonas reinhardtii, Chlorella sorokiniana, Dunaliella tertiolecta and Pseudokirchneriella subcapitata). Algal Res. 2022;1(61):102560.
Acknowledgements
The authors express their gratitude to Prof. Prem Kumar Khosla, Chancellor of Shoolini University of Biotechnology and Management Sciences, Solan, Himachal Pradesh, India; the Shoolini University School of Biotechnology; and the Himachal Pradesh University (Department of Forensic Science), Shimla (H.P.) for providing the technical facilities and laboratory for carrying out trials for antibiotic degradation using microalgae.
Funding
This research received no external funding from any institution.
Author information
Authors and Affiliations
Contributions
The conceptualization of the research was carried out by Sachin Kumar and Kumar Utkarsh. Research planning and manuscript designing was carried out by Ahmad Reza Khan and Ishita Chanana. Original draft preparation was carried out by Sachin Kumar, Ishita Chanana, Azhar Khan, Saurabh Kulshrestha, and Pradeep Kumar. Data curation and figures creation was carried out by Sachin Kumar, Kumar Utkarsh, Azhar Khan, and Navneet Kumar Upadhyay. Manuscript revision was carried out by Sachin Kumar, Ishita Chanana, Azhar Khan, Saurabh Kulshrestha, and Pradeep Kumar. Funding acquisition was done by Azhar Khan, Saurabh Kulshrestha, and Pradeep Kumar. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
We declare that there is no involvement of any human participation, any type of human data, or human issues.
Consent for publication
The authors mutually agree to submit this paper for publication in Algal Research. No other people have been involved in this work.
Conflicts of interest
The authors declare that they have no conflict of interest.
Statement of informed consent, human/animal rights
No conflicts, informed consent, human or animal rights applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kumar, S., Chanana, I., Utkarsh, K. et al. Effect of cephalexin on chlorophyll and carotenoid content of Chlorella pyrenoidosa-2378 and its biodegradation in BG-11 medium. Biotechnol Environ 1, 11 (2024). https://doi.org/10.1186/s44314-024-00011-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s44314-024-00011-4