Abstract
Insects serve as ecosystem engineers in grasslands. Their impacts are comparable in scale to those of mammals, but because they are so much smaller, their roles and influences are not always as obvious. The roles that insects play in grasslands are as diverse as Class Insecta itself, including herbivory, pollination, seed dispersal, soil profile modification, nutrient cycling, parasitism, and serving as intermediaries between plants and wildlife in food webs. In the context of their effects on grassland wildlife species, insects serve as essential food resources for many species of birds, bats, reptiles, mammals, amphibians, fish, and other insects. Insects also have significant effects on the habitat structure available for wildlife because they can, on the one hand, enhance the productivity of grassland vegetation, but alternatively, they have the power to completely defoliate a grassland. From the perspective of food webs, insects play multiple roles. They can serve as food for wildlife, but they also can serve as parasites, vectors of disease, and decomposers. Ecological changes in grasslands due to events such as fire, grazing, herbicide or insecticide application, and habitat fragmentation or loss can affect both wildlife and insects. For that reason, ecologists are often interested in linking the study of a particular wildlife species to the associated insect community. Insects are simply less visible ecological engineers, continually interacting with wildlife, and modifying the habitat where they coexist with wildlife in grassland ecosystems.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
1 General Life History and Population Dynamics
Insects are diverse and abundant within rangelands, including grassland systems. Herein, insects and their ecological interactions in grasslands are addressed. Insects inhabit and occupy the air, soil, vegetation, and aquatic environments. Although they are less conspicuous than other wildlife counterparts, they play a large variety of roles in grassland ecosystems. Their development is affected by humidity, rainfall, and temperature (Kremen et al. 1993). They have high reproductive rates, short life spans, and great mobility in the environment (Porrini et al. 2002), and thus can evolve and adapt quickly to environmental changes. Resultant changes in insect distribution and abundance radiate throughout food webs to impact grassland biota.
Insects provide an incredible array of ecosystem services, from dung burial to pest control, pollination, and food sources (Losey and Vaughan 2006). They modify, aerate, and fertilize the soil, which serves as the foundation of grassland habitat, and serve as herbivores, pollinators, and vectors of disease. Insects, in turn, respond to management of grasslands, and are affected by fire, grazing, herbicide or insecticide application, and habitat fragmentation or loss. A model diagram of how insects affect grasslands would be quite complex. In the most simplistic sense (1) insects affect biotic and abiotic components of grasslands, including virtually all associated grassland species, (2) grassland management affects insects at population, community, and ecosystem scales, and (3) there are a plethora of additional interactions, both direct and indirect, that influence each of these primary relationships. This chapter will highlight some of the most important relationships between insects and plants, soil, other insects, and vertebrates of grasslands, and will explain the role of insects as decomposers and biological control agents. The chapter will also address how grassland management affects these relationships, provide some examples of conservation and management issues, and recommend areas for further research.
1.1 Insects and Plants
The evolutionary history of insects and plants is intertwined, and grasslands can provide a rich habitat for these interactions. Insects provide pollination services to 80% of all angiosperms (flowering plants), and 35% of the world’s food crops depend on animal pollinators (USDA 2022). Many of these co-evolved relationships have become so specialized that in the absence of its pollinator, a plant cannot reproduce. Conversely, in the absence of its food source, many of which are highly specialized, an insect cannot survive. The diversity of flowering plants in a grassland can thus be affected by the corresponding insect community, and vice versa. Floral colors and scents are also a result of this co-evolution (Matthews and Kitching 1984). Grasses are wind pollinated, so they are not dependent on insects for seed production, however, large proportions of grassland communities are forbs, herbaceous flowering plants that are not graminoids (grasses, sedges or rushes). Although the percentage of pollinated forbs in grasslands has not been estimated, it is reasonable that the number is substantial. For context, the estimated proportion of animal-pollinated plant species in temperate zones is 78% (Ollerton et al. 2011). Given that temperate zones include grasslands, shrublands, forest, and other terrestrial communities, it is likely that grassland insects pollinate a large proportion of grassland forb species.
The complex evolutionary history between insects and plants includes a diverse set of interactions, including pollination, herbivory, and parasitism. Insects that consume plants play a variety of functional roles with respect to grasslands, including serving as leaf feeders, leaf miners and gall makers (laying their eggs within the leaf or stem), sapsuckers (feeding on plant juices), and detritivores (feeding on dead plant tissue) (Wilsey 2018). Grasshoppers (Orthoptera) can appear to have a devastating effect on vegetation from an agricultural perspective, but their herbivory also can benefit plant communities because they speed up nutrient cycling by changing the abundance and decomposition rate of plant litter, which increases total plant abundance (Belovsky and Slade 2000). Some insect species consume or parasitize a large variety of plant species, but many insects specialize on utilization of one family of plants or even one species of plant. For example, the grassland obligate regal fritillary butterfly (Speyeria idalia) only consumes Viola species as a host plant and in some regions only one species of violet is consumed, the blue prairie violet, Viola pedatifida (Kelly and Debinski 1998).
The evolutionary pressure of insect herbivory on plants is responsible for the great variety of defensive chemicals plants produce, including nicotine, pyrethrin, and rotenone, which have been exploited for human use as insecticides (Waldbauer 2003). In a complex web of grassland ecosystem interactions, these chemicals influence which insect species can utilize which plant species. In some cases, plant chemicals also make their way into insects (e.g. monarch (Danaus plexippus) butterfly larvae eating milkweed Asclepias spp. (Petschenka and Agrawal 2015)), thus affecting how other vertebrate wildlife may or may not predate upon them. Relationships at the base of the food chain can impact higher levels, affecting wildlife species that feed on insects. Relatedly, these relationships also influence which parasites or diseases may be transmitted from insects to wildlife.
The value of pollination as an ecosystem service has undergone close examination over the past few decades (Losey and Vaughan 2006; Kremen et al. 2007). Although bees (Hymenoptera) are recognized as the most important pollinators, flies (Diptera) are a close second (Larson et al. 2001), and Lepidoptera (butterflies and moths) serve as pollinators for some grassland obligate plant species (Hendrix and Khyl 2000). The associations between flies and flowers are commonly overlooked, but the role of flies in pollination increases with increasing elevation, and flies are important pollinators especially in montane systems. The honey bee (Apis mellifera) is a nonnative insect that is managed to perform pollination services for a broad variety of cultivated fruits, nuts, and vegetables at a continental scale. Honey bees occur in many grasslands and interestingly, native bees can interact synergistically with honey bees to increase the honey bees’ pollination efficiency of crops (Brittain et al. 2013), but there may be potentially negative effects of honey bees on native bees. For example, the presence of honey bees in tallgrass prairie could increase wild bee exposure to viruses (Pritchard et al. 2021) or create competition for resources (Cane and Tepedino 2016). So, knowing which pollinators are most important to conserve in a grassland is more complex than at first glance.
Seed dispersal is another example of the delicate symbiosis between plants and insects. Of all the animals that disperse seeds, only birds and mammals are more important than ants (Hymenoptera). In fact, 35% of angiosperms rely completely on ants for seed dispersal (Waldbauer 2003). Ants gather seeds and carry them back to their nests, dropping some along the way, and discarding the rest in a fertile trash pile just outside their nest. Even this short dispersal distance is advantageous to plants because it lessens the competition between a seedling and its parent and sibling plants (Waldbauer 2003).
Finally, rather than just considering specific co-dependent plant and insect species, it is important to understand the larger community of insects, plants, and other taxa interacting in a grassland. Interactions among plants such as grasses and goldenrod (Solidago sp.) and spiders (Araneae) and grasshoppers (Orthoptera) have been studied extensively in eastern U.S. grasslands. In this system, the presence of spiders can affect which type of plant grasshoppers feed upon, resulting in differential dominance of goldenrod versus grasses. This occurs because predators cause herbivores to suppress the abundance of a competitively dominant plant species that offers herbivores a refuge from predation risk (Schmitz 2003).
1.2 Insects and Soil
Some grassland insects live their lives entirely above ground, whereas other insects spend a portion of their life (usually as eggs or juveniles) in the soil, leaf litter, or aquatic environments and a different portion of their life above ground. Another group of insects spend most of their time living in and on the grassland soil and physically modify the soil profile, improving the habitat for plant growth (Lee and Wood 1971). For example, termites (Blattodea) and ants redistribute soil and nutrients, bringing mineral-rich material from lower soil layers and mixing it with upper layers with high organic matter content, creating a fertile environment rich in carbon, nitrogen, and phosphorus supporting plant growth (Lee and Wood 1971; Waldbauer 2003). Termites and ants build tunnels increasing soil porosity, which facilitates root growth, aeration, and water storage and drainage (Waldbauer 2003). Insects such as springtails (Collembola) and termites shred plant materials into smaller fragments that can be used by bacteria and fungi (Wilsey et al. 2005; Wilsey 2018). Without these types of insect activity, nutrient cycling would be lessened, reducing plant productivity of grasslands. Dung beetles feed on animal excrement as both adults and larvae, thus fertilizing the soil in which they live (Nichols et al. 2008). In addition, without insect activity, lower quality, less fertile soils would become exposed at the surface (Lee and Wood 1971).
In turn, insects are affected by soil conditions. For example, De Bruyn et al. (2001) found that species richness and diversity in flies were affected by soil pH, soil moisture, and the amount of organic matter present in the soil. As such, there was significant feedback between the insect community and grassland soil, and these interactions affected the structure, composition, and nutrient quality of vegetation available for plants and wildlife.
1.3 Insects and Wildlife
As noted previously, insects provide a vital connection between plants and wildlife. Herbivorous insects, “play an indispensable and pivotal role as intermediaries in food chains by making the nutrients synthesized by plants available to animals that do not eat plants” (Waldbauer 2003). Insects serve as food for birds, bats, reptiles, amphibians, and fish (Goulson 2019). In aquatic environments, terrestrial invertebrates, most of which are insects, can be a significant source of prey for fish, sometimes providing about 50% of their annual energy (Saunders and Fausch 2007). Similarly, grassland birds and mammals in terrestrial habitats adjacent to aquatic environments harvest rich insect food sources from aquatic and terrestrial environments. As summarized by Malmqvist (2002: 688), “Aquatic insects subsidize terrestrial birds (and other terrestrial predators such as bats, spiders and predacious insects) and terrestrial insects subsidize fish production in the stream habitat.”
Grasslands, specifically, produce abundant insects offering a rich food source for wildlife. Kaspari and Joern (1993) conducted a study of three grassland bird species (grasshopper sparrows [Ammodramus savannarum], lark sparrows [Chondestes grammacus], and western meadowlarks [Sturnella neglecta]) in the Nebraska Sandhills and found that grasshoppers and small beetles (Coleoptera) were the primary food source, but these species also consumed other invertebrates, including Homoptera (aphids, scale insects, cicadas, and leafhoppers), Hymenoptera (bees, wasps, ants, and sawflies), and Araneae (spiders). Prey selection in this group of grassland birds was found to be a complex function of prey size, energy, and other nutrients. Most quail, grouse (Tetraoninae), and pheasant (Phasianus colchicus) chicks also rely on insects as a source of protein (Losey and Vaughan 2006). For waterfowl, 43% of species are primarily insectivorous (Ehrlich et al. 1988). With respect to raptors, Swainson’s Hawks (Buteo swainsoni) prey on both insects and mammals, and grasshoppers can comprise a “staple sustenance” of their diet (Cameron 1913). Additionally, insects “bridge the size gap between large predators and unicellular plants or animals too tiny to be profitable eaten by a large animal” (Waldbauer 2003). Without insects, many food chains, including those in grasslands, would collapse.
1.4 Insects as Decomposers
Grassland insects aid crucial nutrient cycling by consuming carrion and decomposing organic matter. In this process, dead organic matter is returned to the soil as minerals and to the atmosphere as gases (Waldbauer 2003). In grasslands that support large populations of mammals, decomposition of carrion and feces by insects is significant and if ceased this understated ecosystem service would quickly become apparent. In Colorado, De Jong and Chadwick (1999) reported 53 insect taxa utilizing decaying rabbit (Oryctolagus cuniculus) carcasses. Carrion beetles (Silphidae) are especially important in decomposition. For example, Sikes (1994) found that over 50 species of carrion beetles were heavily dependent on the ungulate carcasses present in sagebrush steppe of the northern range of the Greater Yellowstone Ecosystem.
In grasslands that are grazed by cattle (Bos taurus), each animal can produce over 9000 kg of solid waste per year (Losey and Vaughan 2006). Beetles in the family Scarabaeidae provide the ecosystem service of decomposing and burying this waste, which reduces the habitat available to parasites such as flies, thus reducing cattle losses due to horn flies (Haematobia irritans irritans) and face flies (Musca autumnalis) (Losey and Vaughan 2006). In a related manner, the removal of dung beetles (Scarabaeinae) in some grasslands has been experimentally shown to decrease plant productivity (Wilsey 2018). Without ungulates, many species of beetles could not survive, and without beetles, carcasses and feces would decompose much more slowly, changing the rate of nutrient cycling and productivity in grasslands.
1.5 Insects as Biological Control Agents
Decomposition by insects also serves as an example of how insects act as biological control agents. Insects can serve as biological controls on other insects, plants, and mammals. One insect species can affect the abundance or distribution of another insect species through competition, predation, parasitism, or mutualism. Destructive outbreaks of insects often occur when these relationships have been disrupted by human activity (LaSalle and Gauld 1993). Insects also can keep “pesky” plants at bay, both by herbivory pressures and seed predation. For example, insects have been used to slow the spread of nonnative plant species like leafy spurge (Euphorbia esula) in the Northern Great Plains (Butler et al. 2006), prickly pear cactus (Opuntia) in Australia, and St. John’s wort (Hypericum perforatum) in California (Waldbauer 2003). Wildlife population growth can be affected or controlled by insects through insect-borne diseases, parasitism (Mooring and Samuel 1998), and competition for plants if both the insect and the wildlife species are herbivores (Waldbauer 2003). Again, these relationships can be direct or indirect. As an example of both a direct and an indirect relationship caused by one insect species, ticks (Ixodida) may cause significant blood loss to their wildlife host species (direct effect) and influence the foraging ability of elk (Cervus canadensis), bison (Bison bison), and moose (Alces alces) (indirect effect) (Mooring and Samuel 1998).
2 Species and Population Status Issues
2.1 Historical Versus Current Distributions, Conservation Status
A number of butterfly, bee, and beetle species that historically occurred on U.S. rangelands are now listed as threatened or endangered. Some examples of these species are listed in Table 26.1. However, due to the sheer number of insect species, the historical knowledge of insect species distribution patterns is limited compared to plants or vertebrates. A broad estimate of 800,000 insect species have been named worldwide and, for the majority of these species, the scientific community knows relatively little about their biology, distribution, or abundance (Goulson 2019). There is such a dearth of knowledge that scientists are challenged to assess and quantify even the crudest measures of changes in diversity and distribution over time limiting assessment of conservation status.
Some prominent grassland insect declines across the U.S. have been documented by the U.S. Fish and Wildlife Service (USFWS 2022). We provided examples of endangered or threatened insects under the Endangered Species Act (1973; ESA) and those having state-level designation as “Species of Conservation Concern” (Fig. 26.1 and Table 26.1). The rusty patched bumble bee (Bombus affinis) was listed as endangered in 2017 (Lambe 2018). For many of these insects, habitat loss and/or modification are primary threats (USFWS 2022). Conversion of grasslands into row crops has reduced tallgrass and shortgrass prairie upon which many grassland insect species are dependent (see Chaps. 3, 5). The use of fire and/or grazing, can also have detrimental effects, depending upon the insect species (Kral et al. 2017). The endangered American burying beetle (Nicrophorus americanus) now occurs in less than 10% of its former range in the grasslands in eastern Oklahoma, central and southern Nebraska, southeastern Kansas, and southcentral South Dakota, but there are multiple possible reasons and the main drivers of decline remain unclear (Sikes and Raithel 2002).
Selected rare, threatened, and endangered insects of North American grasslands. a The American burying beetle, Nicrophorus americanus, is threatened in the shortgrass prairie ecoregion and also in the sandhills of Nebraska. Photo source Doug Backlund. b The regal fritillary butterfly, Speyeria idalia, is a Species of Conservation Concern in tallgrass and shortgrass prairie ecoregions. Photo source Raymond Moranz. c The rusty patched bumble bee, Bombus affinis, is endangered and occurs in the tallgrass prairie ecoregion. Photo source https://www.fws.gov/midwest/endangered/insects/rpbb/rpbbid.html
In addition to species formally listed as endangered or threatened under ESA, there are many rare insects that have been proposed for listing but have not yet received status designation. The regal fritillary (Speyeria idalia) butterfly has been a “Species of Conservation Concern” in Midwestern grasslands for several decades. It has been lost from much of its historical distribution in Midwestern prairies due to habitat loss and fragmentation (Kelly and Debinski 1998). The monarch butterfly (Danaus plexippus) has more recently become a Species of Conservation Concern, both in terms of its eastern and western populations, and this concern has recently advanced to the federal level. The western population dropped by 97% of their average historic abundance between the 1980s and mid-2010s, and during 2018–2019, the population plummeted even farther, to fewer than 30,000 monarchs (Pelton et al. 2019), but then rebounded to 200,000 in 2021–2022 (McKnight 2021). The decline of monarch butterflies in the eastern U.S. has been attributed to herbicide effects and habitat conversion on host plants, poor weather, insecticide exposure and reduced overwintering habitat (Belsky and Joshi 2018). Although the monarch was proposed for listing as a threatened species, a 2020 ruling determined that listing was “warranted but precluded,” meaning that the U.S. Fish and Wildlife Service does not have enough resources to complete the listing decision process because of higher-priority reviews (USFWS 2020). However, on June 21, 2022, the International Union for the Conservation of Nature (IUCN) listed migrating monarchs as Endangered on the IUCN Red List (IUCN 2022).
Recent assessments of insect decline at a global level and in regions outside of North America can be used to inform grassland insect conservation within North America (Hallmann et al. 2017; Goulson 2019; Zattara and Aizen 2020). For example, Zattara and Aizen (2020) reported that the number of bee species being collected or observed over time has declined since the 1990s and that these results might, in part, reflect increased impediments to specimen collection and data mobilization, as well as reduced sampling coverage. However, this also could reflect a worldwide decline in bee diversity given that many species are becoming rarer. Similarly, Forister et al. (2021) documented a 1.6% annual reduction in the number of individual butterflies observed over the past four decades in 70 locations within the western U.S. In an alternative approach to species abundance assessments, some scientists have measured trends in total insect biomass (Hallmann et al. 2017). Even these relatively crude assessments reveal declining trends, such as a recent study that reported a 75% decrease in total flying insect biomass over a 27-year period in protected areas of Germany (Hallmann et al. 2017).
Systematists have pointed out that the limited number of taxonomists available to identify many of these species may be another factor influencing these trends in insect abundance, which would make interpretation of declines in species less robust. It is well recognized that a limited number of taxonomists and related jobs is a major challenge (Agnarsson and Kuntner 2007), potentially affecting scientists’ ability to assess insect trends. Determining the drivers and the relative significance of these purported temporal trends in insect biomass deserves additional attention in future research.
Changes in insect populations can be reflected in other trophic levels of the ecosystem, and thus have significant relevance to grassland wildlife. In some cases, monitoring insect populations allows the prediction of effects on organisms in higher trophic levels (LaSalle and Gauld 1993). Changes in insect populations also may precede changes in lower trophic levels (Erhardt and Thomas 1991) and provide information about changes in habitat. For example, honey bees have been used as environmental monitors for decades (Devillers 2002). Carabid beetles (Coleoptera, Carabidae) have been used to document long-term (e.g., 100+ yr.) changes in habitats (Turin and Den Boer 1988). Fossil records of arthropod communities even have been used to construct climate histories (Atkinson et al. 1987).
2.2 Population and Community Monitoring
A diverse set of field and analytical approaches are used to monitor populations and communities of insects in grasslands. The statistical approaches used for insect population and community analyses are diverse and similar to those used for wildlife species. Numerous books have been devoted to estimating population sizes (Borchers et al. 2004; Mills 2012; Buckland et al. 2015) and analyzing ecological community structure (Magurran 1988; Mittelbach 2018), so these topics will not be covered in this chapter. However, in some cases the methods of data collection for population and community assessment of insects differ from wildlife methods. Below are summarized some of the most frequently used methods for insect population and community monitoring (sweep netting, pan traps, pitfall traps, and individual species netting). Similar to wildlife surveys, video monitoring and quadrat surveys can also be used for insect surveys (Zaller et al. 2015) but are not described here given their less frequent application.
2.2.1 Sweep Netting
The most common approach to monitoring insect communities is through sweep netting. Sweep netting is a consistent and reliable survey tool for capturing vegetation dwelling arthropods. This technique is particularly good for medium sized insects but can be challenging for collecting smaller insects. Spafford and Lortie (2013) note the value of sweep netting for Thysanoptera (commonly known as thrips), infrequently collected (i.e., rare) insects, and Arachnida (spiders, ticks, mites, and harvestmen). Other methods may need to be used in combination with sweep netting to assess the entire grassland community such as ground dwelling arthropods.
Although dimensions may vary, one example of a sweep net has a sturdy canvas net bag attached to a 38 cm (15 inch) diameter ring and a wooden handle 91.5 cm (3 feet) long and 2.5 cm (1 inch) in diameter. Sweep nets are a much more substantial tool than the aerial net used for individual insect surveys described below. For standardized sampling, sweep netting involves a surveyor taking a specific number of steps through the grassland with a canvas sweep net and swinging the net broadly from side to side across ~180° with each step as they walk along a transect of designated length. The number of swings and the distance of the transect are generally standardized so that effort is constant across spatial or temporal replicates. At the end of the transect, the observer grabs the net and closes it with their hand and then carefully turns the netting inside out, placing their catch into a receptacle such as a clear plastic bag. The plastic bag can then be inflated to reduce the chance of crushing the insects. The sample is often taken back to a cooler and then stored in a refrigerator or freezer until the insects can be sorted for identification. With sweep netting, there is often quite a bit of time spent removing the insects from the plant material before the insects can be identified to family, genus, or species with the aid of a dissecting microscope. Alternatively, if the investigator is seeking a particular type of insect group such as bees or ants, these insects can be taken from the sweep net and individually placed in small glass vials filled with alcohol as a preservative.
2.2.2 Pan Traps
Pan traps or “bee bowls” are colorful plastic bowls (usually white, yellow, and blue, which colors are visible to bees) filled with a soapy water mixture used to passively sample insects (Baum and Wallen 2011). Pan traps are particularly good at capturing bees and flies that are collecting nectar on flowers, but they also catch a broad variety of insects traveling in the same area. The colors of the bowls mimic the colors of the flowers blooming in the grassland. The bowls can be attached to sticks or posts at various heights within and above the vegetation to select for bees that fly at a particular height, or they can be simply laid on the ground. The height of the pan is set to target the insects moving either through or above the vegetation. However, it is important to note the sampling bias in pan trapping; some of the larger-bodied insects (e.g., bumble bees [Bombus spp.], grasshoppers) are less likely to be captured. There is also an issue that pan traps may undersample bee species richness and abundance when floral resources are abundant because bees go to the flowers rather than to the bowls, which can bias estimates of species richness and abundance (Baum and Wallen 2011). Finally, if the traps do not contain a preservative, they will get stinky on warm summer days if not collected within 24 h or less. In such cases, a preservative like propylene glycol be added to the soap water, or traps can be sampled with greater frequency to collect specimens.
2.2.3 Pitfall Trapping
Pitfall trapping particularly selects ground-dwelling insects, such as ants, beetles, and a broad range of hopping and walking insects as well as spiders. Note that spiders, although not insects, are often included in the context of insect community surveys. This technique involves digging a small hole in the ground and placing a receptacle, such as a plastic cup, flush with the ground (Zaller et al. 2015). An efficient modification is to insert two cups (e.g., Solo Cups) inside one another and bury them flush to soil surface. When collecting samples, only the inner cup needs to be removed and the dirt hole remains undisturbed. Soapy water, or if a preservative is desired, water mixed with antifreeze, is placed in the cup. Use of a preservative is often preferable in warm environments. Propylene glycol (regular automotive antifreeze) or RV antifreeze are two options; the latter is non-toxic containing alcohol as a preservative. Just water can also be used to avoid captured insect mortality, but traps should be checked relatively frequently. When insects walk by, they fall into the cup and get stuck in the fluid. A consistent diameter of trap should be used among sites so that there is no capture bias and so that comparisons among sites can be valid. Many of the insects that walk in the vicinity of the pitfall trap may not be captured, so if needed additional methods for insect community monitoring should be considered (Zaller et al. 2015).
Similar to pan traps/bee bowls, pitfall traps need to be checked within a day or two or the insects may become unidentifiable. If a preservative is added (e.g., 1:1 propylene glycol:water mix) traps can be left deployed for up to a week. However, sampling periods should be carefully considered because when open pitfall traps and bee bowls continuously collect insects risking oversampling. The liquid and trapped insects are generally collected in small plastic bags and taken to a lab for identification. Refrigerated storage is recommended until samples can be processed.
Generally, if objectives are to sample ground-dwelling arthropods, pitfall traps provide good samples when deployed correctly and efforts are taken to control bias. Leading Coleoptera and Arachnid scientists commonly use pitfall traps to assess community structure around the world. When pitfall traps are combined with sweep nets and/or pan traps, valuable data can be collected to assess insect community structure.
2.2.4 Individual Species Netting and Observation
Individual species of butterflies, bees, or dragonflies (suborder Anisoptera) can be surveyed with aerial nets (generally 38.1–45.7 cm [15–18 inch] diameter ring, and 122 cm [48 inch] or longer handle length). Aerial nets are much more lightweight than sweep nets, usually with aluminum handles and lightweight netting material. The netting is somewhat transparent and allows the surveyor to net the insect, handle it carefully within the net, extract it with forceps, and either collect it in an envelope (e.g., butterflies), a cyanide jar (bees), or in a small vial of alcohol (other larger-bodied insects), or release it unharmed. Individual species-focused netting can be used to detect species presence or to conduct mark-recapture surveys to monitor the size of an insect population. For mark-recapture, the insect is carefully handled and, depending upon the type of insect, the wings or body can be marked with a permanent marker or a small sticker. Such markings, if done properly, can have no adverse effects on the insect and, in the case of butterflies, individuals can be released and recaptured multiple times during their lifetime (Auckland et al. 2004).
Finally, there is a growing movement towards the use of visual observation rather than collection for more easily identifiable species such as butterflies and bumble bees. For these easily identifiable species, we are learning a lot about distribution and status trends through well designed observational studies, in many cases using community, amateur, and volunteer scientists to collect data such as the Nebraska Bumble Bee Atlas (Xerces 2022).
2.2.5 Analytical Approaches
Statistical analyses for population and community ecology research on insects are similar to those used by wildlife biologists, as noted in Sect. 26.2.2. However, for insects, there is the additional challenge of accounting for populations capable of large interannual fluctuations, including species such as painted lady butterflies (Vanessa cardui) (Vandenbosch 2003), grasshoppers (Kemp 1992), southern pine beetles (Dendroctonus frontalis) (Turchin et al. 1999), eastern spruce budworm (Choristoneura fumiferana) (Zoladeski and Maycock 1990), and cicadas (Cicadoidea) (Cook et al. 2001). Given these natural fluctuations, changes in numbers—even dramatic at times—are not necessarily an indication that major long-term population-level changes are underway. However, the loss of a subset of the insect community, a major change in geographic distribution patterns, or a downward turn in multiple grassland insect species that exhibit similar sensitivities could be cause for concern. For example, a meta-analysis of species range shifts might detect poleward shifts in geographic distribution patterns associated with climate change (Parmesan et al. 1999). Furthermore, when scientists evaluate insect responses to environmental change, some insect taxa are more sensitive than others. In some insect community analyses, certain species can be classified as either disturbance-tolerant or habitat-restricted (Ries et al. 2001). A large increase in the disturbance-tolerant species or the disappearance of habitat-restricted species would warrant investigation. Habitat-restricted species can be especially valuable indicators when habitat loss or fragmentation is an issue. And, notably, some insects have very short dispersal distances despite that fact that they are winged, making them more vulnerable to habitat loss than might be assumed.
3 Habitat Associations
3.1 Historical/Evolutionary
As described in the introduction to this chapter, insects have a plethora of specialized ecological roles that they play, whether they live in or on the vegetation, on or within the soil, or within the air or the water. All these associations within a grassland habitat have impacts on grassland wildlife species. And as described in previous chapters, the effects of grazing, fire, and mechanical management have changed the character and ecology of grasslands at large spatial scales.
3.2 Contemporary Grasslands
The grasslands that once covered North America have been converted by row crop agriculture, extractive industries, urbanization or impacted by raising domesticated livestock (see Chap. 1). As fragmentation and loss of grasslands becomes a predominant regional driver, keystone wildlife species can be lost, and broad suites of other organisms associated with the ecosystem are affected, including insect communities. In some regions, grasslands are being restored, fire and grazing regimes are being returned, and models to affect such change include both ecological and sociological approaches (Miller et al. 2012). However, sometimes even if the ecosystem looks like a native grassland, it may not yet act like a native grassland. For example, restored grasslands (grasslands that are replanted after the native vegetation has been modified or lost due to tilling, development, herbicide use, etc.) can have very different seasonal patterns of abundance of floral resources for pollinators as compared to native grasslands (Delaney et al. 2015). Given that pollinators are dependent upon floral resources for growth and reproduction, such differences in the amount and timing of resource availability could have real consequences on grassland insect abundance and diversity.
Contemporary grasslands frequently contain combinations of native and nonnative species of grasses, remnants of unplowed prairie and restored areas, and forbs and woody plants. These changes in grassland composition can be a result of inter-seeding (seeding within a grassland to enhance forage production or reduce erosion), invasive plants, tillage, grazing, fire, herbicide or fertilizer treatments, and many other forms of management. Resulting differences in the plant community can in turn affect the stature of the vegetation, the ratio of forbs to grasses, and the amount of woody vs. non-woody vegetation. Nonnative plant species can alter the amount of bare ground, the amount of litter that remains at the end of the growing season, and how the vegetation responds to fire. Similarly, the amount of bare ground versus litter cover can affect which insect species inhabit a grassland due to their needs for nesting, overwintering, etc. For example, McGranahan et al. (2012) found that tall fescue (Lolium arundinaceum), a grass that is commonly seeded into grasslands in the Midwestern U.S., creates patches of living grass in the early spring within a grassland and limits the ability for fire to spread. Similarly, inter-seeding of grasslands with plant species such as tall fescue can create sweeping effects on plant–herbivore interactions and energy flow through the food web because tall fescue often harbors a fungal endophyte that modifies food web interactions (Rudgers and Clay 2007). Tall fescue, when consumed in large enough quantities, also can be toxic to livestock (Paterson et al. 1995) and may affect wildlife, but less research has been conducted on the latter.
4 Rangeland Management
A broad set of rangeland management tools (grazing, fire, and mechanical approaches) have been deployed to manage grasslands in a variety of ecoregions across North America. These tools can have long-lasting legacies, and the history of such management can affect the vegetation composition of the grassland long after a management tool was applied (Moranz et al. 2012). Additionally, the effects of one type of management cannot be expected to result in the same vegetation response for all grasslands. Vegetation composition and the history of previous management can affect how a grassland will respond to management (McGranahan et al. 2012). For example, as noted in Sect. 26.3.2, the presence of an invasive grass may make it more difficult for a manager to apply fire as a management tool.
4.1 Livestock Grazing
As previously noted, grasslands evolved with herbivory and not just by large mammals, but also insect herbivory. Mammalian grazing, whether accomplished by domesticated livestock or wildlife affects the grassland habitat available for insects.
Insect communities can and do have variable responses to grazing and this variation in response also can be influenced by the ecoregion. For example, grassland insect communities in the western deserts of Arizona, which did not evolve with bison herbivory, were found to be sensitive to cattle grazing (Debano 2006). Coleoptera (beetles) had lower species richness, Diptera (flies) were less diverse, and Hymenoptera were less rich and diverse on livestock grazed sites but Hemiptera (true bugs) were more diverse on livestock grazed sites (Debano 2006). In contrast, a grazing study in the shortgrass prairie of central Montana with cattle (Goosey et al. 2019) found that ground-dwelling arthropods that served as bird food (Coleoptera, Lepidoptera, Hymenoptera and Orthoptera) were twice as prevalent in cattle grazed pastures as in ungrazed pastures. Meanwhile, pastures ungrazed by cattle had twice the activity-density (number of beetles that cross the perimeter of the trap opening in a given time (Kromp 1989)) of ground-dwelling arthropods, which was largely driven by increases in detritivores and predators (Goosey et al. 2019). In seeking generalities among livestock grazing studies, plant community, geographic location, as well as the stocking rates, season of use, and grazing regime can all affect insect responses. Similarly, species level results may be different compared to the findings of family level analyses.
In some cases, livestock grazing has been proposed as a tool to control insects, such as grasshopper populations in rangelands. O’Neill et al. (2010) found general support for the hypothesis that grazing could be used to reduce pest grasshopper densities, but there was variation in how specific grasshopper species responded to grazed versus ungrazed treatments of Agropyron spicatum/Poa sandbergii pastures in southwestern Montana shortgrass prairie depending upon site, year, and vegetation assemblage.
The intensity and duration of livestock grazing can have effects on adjacent aquatic communities. For example, grassland stream fish communities can be affected when vegetation structure adjacent to streams is modified by grazing so that fewer insects fall into the streams as food for fish. In a study of trout streams in the Wyoming Basin that compared two types of livestock grazing during the summer months (high-density, short-duration grazing versus season-long grazing; see Chap. 4 for grazing-system definitions), the input of terrestrial invertebrates to the riparian areas was two to three times greater in areas managed with high-density, short-duration grazing due to more overhanging vegetation than those managed with season-long grazing management (Saunders and Fausch 2007). Effects of changes in fish communities could impact bird and mammal communities that prey on these fish.
In addition to the intensity of grazing, the combination of grazing intensity and precipitation can create heterogeneity in vegetation structure. Newbold et al. (2014) found that insect responses to grazing in the shortgrass prairie of Colorado were more pronounced in a year when spring and summer rainfall was low, noting that “both exclusion from grazing and precipitation are presumably necessary to create pronounced differences in vegetation structure to which arthropod consumers then respond.”
4.2 Fire
The effects of fire on both grasslands and insects are diverse, including direct and indirect effects, seasonality, frequency, and fire-grazing interactions. Because grasslands evolved in the context of fire, burning can, in some cases, be effectively used to manage invasive plant species (DiTomaso et al. 2006) and enhance flowering of forb species (Goldas et al. 2021) with potential to indirectly affect insects. Fire is not generally used to directly manage insects, but the effects of fire on insects are often studied along with using fire as a grassland vegetation management tool (Schlicht and Orwig 1998; Kral et al. 2017). A literature review (Kral et al. 2017) of insect responses to fire found that some orders tended to respond negatively (Araneae, Lepidoptera) or positively (Coleoptera, Orthoptera) to fire, but that responses were highly variable among taxa and that characteristics such as life stage, feeding guild and mobility of the insects are key in predicting responses.
The effects of fire in the context of insects can be divided into two categories: (1) the direct effects on the insects (i.e., incineration) and (2) the indirect effects that manifest themselves in the insect populations via the effects on the vegetation that insects use (Fig. 26.2) (Vogel et al. 2010). With respect to direct effects, for any species of insect that predominantly occurs above ground, fire has the potential to directly cause an immediate decrease in the insect population (Vogel et al. 2010). For this reason, there are more management recommendations about the frequency of management for fire as compared to other types of grassland management. Insects may eventually return to near pre-burn levels as vegetation recovers and soil litter stabilizes, but response time can vary. Swengel (2001) noted that many insects decline markedly immediately after fire, with the magnitude of reduction related to the degree of exposure to the flames and mobility of the insect populations. Species that live underground, such as ants, burrowing beetles, or insects overwintering underground may suffer fewer, if any, direct effects of a fire if the fire occurs when they are underground, but this field of inquiry has not been extensive, and the degree of fire effects may vary with fire intensity. Fire had no population or diversity level effects on underground arthropods in one tallgrass prairie study (Pairis et al. 2003), but the lack of impacts could have been due to post-burn recolonization.
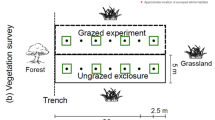
A path diagram depicting direct and indirect relationships between time since burn, vegetation characteristics, and butterfly abundance for butterflies in Iowa grasslands. Independent variables chosen for inclusion in the path models were: floral resources (number of flowering ramets), warm-season grass (percent cover of warm-season grasses), and bare ground (percent cover of bare ground). Residual from the model is designated as e1. Reproduced from (Vogel et al. 2010)
Most fire studies focus largely on the response of insects to changes of vegetation post-fire, i.e., indirect effects. The most dramatic change to vegetation composition is the elimination of the above-ground foliage. Fire changes the composition of the vegetation dramatically by reducing above-ground biomass, removing woody vegetation, releasing nutrients, and increasing the amount of sunlight that reaches the soil (Radho‐Toly et al. 2001). Fire also can stimulate the growth of fire-related annual forbs and grasses (Korb et al. 2004). For insect families associated with forbs and grasses, such as Acrididae (grasshoppers, locusts), Gryllidae (crickets), Tettigoniidae (katydids), Aphididae (aphids), Margarodidae (mealybugs), Chrysomelidae (leaf beetles), Carabidae (ground beetles), Cantharidae (soldier beetles), Coccinellidae (ladybird beetles), Asilidae (robber flies), Meloidae (blister beetles), Formicidae (ants) and Lepidoptera (butterflies, moths, cutworms, army worms, skippers), temporary increases in populations, may occur as a result of the increased forb productivity after a fire.
The seasonality of a fire also can have profound effects on the way that environmental change manifests itself in the insect community. Generally, late spring burning of grasslands is thought to reduce insect populations more than early spring burning (Higgins et al. 1987), but species-specific responses may vary. For example, with respect to grasshoppers, Knutson and Campbell (1976) found that early spring burning in Kansas grasslands caused grasshoppers to emerge three weeks earlier than normal, and grasshoppers were higher in number the second year following an early burn. Mid-spring burning produced fewer grasshoppers than early burning, and late spring burning produced fewer grasshoppers than mid- or early spring burning, potentially due to effects on a particular portion of the life-cycle.
The frequency of fire is also critical to the insect response. Frequent or even annual burning of grasslands is part of the culture in some North American grasslands, such as the Flint Hills of Kansas in the tallgrass prairie ecoregion. Welti and Joern (2018) found that fire frequency affected flowering plant and floral visitor community composition in the Flint Hills. In the same system Welti et al. (2019) found that changes in fire frequency affected plant and grasshopper community composition but did not have significant main effects on the plant–grasshopper network structure. The effect of repeated burning on soil arthropods in a Wisconsin tallgrass prairie was studied by Lussenhop (1976), where burning of re-established tallgrass prairie that had been burned biannually for decades was continued for two more burns on one area and discontinued on the other and as a control, a third area was raked to remove the litter. First-year results showed no significant difference in soil microarthropods, but by the fourth year the unburned areas had significantly fewer herbivorous and carnivorous insect species than the burned and control (raked) areas. Lussenhop (1976) concluded that the unburned area was less productive, causing a decrease in soil microarthropods.
Davies et al. (2014) found that in big sagebrush communities (Artemisia tridentata Nutt. ssp. vaseyana (Rydb.) Beetle) on the Columbia plateau of Oregon burning altered the arthropod community, which included a doubling of the density of arthropods in the first post-burn year. Some specific groups of arthropods increased, and others decreased with burning. Notably, Hemiptera were 6.6- and 2.1-fold greater during one- and two-years post-burn compared with the control. Changes in the insect community were associated with increases in plant diversity in burned sites in the first post-burn year, but that difference was gone by year two and burned plots actually had lower plant diversity by the third post-burn year (Davies et al. 2014). Changes as a result of fire can have mixed effects on wildlife, in some cases providing additional insect food resources to greater sage-grouse (Centrocercus urophasianus), sagebrush lizard (Sceloporus graciosus), northern horned lizard (Phrynosoma platyrhinos platyrhinos), and sage thrasher (Oreoscoptes montanus) (Davies et al. 2014).
Finally, the combination of fire and grazing management also can affect insect abundance and distribution patterns because this combination can create heterogeneity in vegetation structure across the landscape. For example, shorter and sparse vegetation may be found in recently burned patches and taller, denser vegetation in less recently burned patches. Patch-burn grazing, a way of managing a pasture to rotate the patch that is annually burned, has been extensively examined with respect to vegetation and bird responses to landscape heterogeneity (see Chap. 4), and in some cases with respect to insect responses (Debinski et al. 2011). The models of Fuhlendorf et al. (2009) that examine how bird species respond to patch-burn grazing management also can be applied to the insect community. For example, Moranz et al. (2012) found that butterflies in tallgrass prairies such as the regal fritillary, wood nymph (Cercyonis pegala), and monarch are more likely to be found in tall-stature grasses, which can be associated with longer intervals between burns, whereas the habitat generalist eastern-tailed blue (Cupido comyntas) is more likely to be found in short-statured grasses. In comparing ant species distribution on three combinations of grazed and burned pastures (patch-burn grazed, graze-and-burned, and burn-only), Moranz et al. (2013) found that “opportunist” and “dominant” ant species in tallgrass prairies were more abundant in burn-only tracts than grasslands that also had grazing treatments. Generalist ant species were more abundant in graze-and-burn tracts than in burn-only tracts. Abundance of Formica montana, the dominant ant species, was negatively associated with time since fire, whereas generalist ant abundance was positively correlated with time since fire.
In summary, there are many plant–insect interactions within a grassland ecosystem that can be affected by the seasonality and frequency of fire as a form of grassland management. Responses of particular insect species depend upon their natural history, where they live in the grassland, how specialized they are on particular plants, and how they overwinter. Some insect species evolved to take advantage of recently burned landscapes while others evolved to advance in older, more mature, landscapes. As such, sweeping generalizations about insect species or insect taxonomic group responses to fire are often limited in accuracy.
4.3 Herbicide, Pesticide, and Mechanical Treatments
Many grasslands are threatened by the encroachment of woody species and nonnative grasses and forbs, and herbicides are often used to reduce or remove occurrences of these plant species. Chemicals used to control certain plants can also significantly affect the insect community, albeit indirectly. As an example, Taylor et al. (2006) found that weedy plots on a Montana experimental farm contained 12 times more biomass of common insects eaten by nestling birds compared to monoculture plots prior to spraying, but following spraying with bromoxynil and imazamethabenz herbicides, weedy plots contained only 3 times more biomass than monoculture plots. From a wildlife perspective, rangeland modification associated with herbicide application has the potential to have significant effects, particularly for birds, due to the associated changes in the vegetation structure and composition. There is not adequate space in this chapter to thoroughly cover herbicide treatment as a form of grassland management, but in the context of insect changes in the plant community due to herbicides, such chemicals will undoubtedly affect the insect community.
Insecticides are not as frequently used directly on grasslands, but there are some examples that relate to both insect and wildlife responses. In Wyoming, > 1.7 million ha were treated with toxaphene and chlordane for grasshopper control during 1949–50; during the two years afterwards, pesticide poisoning was suspected in 11 of 45 Greater Sage-Grouse mortalities (Rowland 2019). From a landscape context perspective, native grasslands are often adjacent to agricultural lands where insecticides are extensively used. As such, the topic is introduced here, but covered more substantially in Sect. 26.6.2.
Effects of mechanical treatments have not been as extensively examined in terms of insect responses except in the context of being a replacement for fire or grazing. For example, haying is, in some grassland studies, examined as the “third wheel” of management (see Chap. 5). More frequently, the comparison treatment is “ungrazed” or “idled” (not cut, burned, cropped, heavily grazed, cultivated, or otherwise disturbed). In sagebrush systems, mowing and “chaining” (where a large heavy chain is dragged over the ground to clear vegetation) have been used to reduce woody vegetation and increase grasses. There are some studies of chaining on the responses by birds (Castrale 1982), but there are no studies of the effect of chaining on insects in rangelands. Studies of the effects of mowing on bird responses provide some insight into these management effects on insects. For example, Hess and Beck (2014) found that mowing in the Wyoming Basin did not improve Greater Sage-Grouse nesting or early brood-rearing habitat attributes such as cover or nutritional quality of food forbs, or counts of ants, beetles, or grasshoppers. There are ample publications that evaluate responses of insects to grassland mechanical treatments at the fine-grained scale of tens of hectares (Debinski and Babbit 1997; Pairis et al. 2003) but excluding a small number of studies such as Stoner and Joern (2004), studying effects of mechanical treatments on insects at a landscape scale is an area ripe for additional research.
5 Insects and Disease
A complete investigation of diseases that affect insects is beyond the scope of this chapter. However, some of the major diseases in which insects serve as vectors of disease to domesticated animals and wildlife are described below.
5.1 Insects as Vectors of Disease
Entire books and sections of books have been written about the impacts of insects as vectors of disease on livestock and wildlife (Eldridge et al. 2000; Capinera and Capinera 2010; Botzler and Brown 2014). Suffice it to say that insects can cause a broad set of diseases in livestock and similar relationships apply to many wildlife species. To list a few prominent examples, the following insects are major issues for livestock: black flies (Simuliidae), biting midges (Culicoides), horn flies (Haematobia irritans), stable flies (Stomoxys calcitrans), horse flies (Tanabidae) and deer flies (Chrysops) as well as mosquitos (Culicidae), face flies, cattle grubs (Hypoderma), lice (Anoplura and Mallophaga), screwworms (Cochliomyia hominivorax), ticks (Ixodida), and mites (Demodex bovis) (Steelman 1976). These insects can have minor to major impacts on the health and productivity of livestock, affecting their skin, eyes, milk production, weight gain and weight loss, and serving as vectors of disease (Steelman 1976).
Wildlife populations also can be affected by insects as vectors. For example, heavy tick infestation can have significant impacts on moose reproduction and survival of young (Ellingwood et al. 2020). Biting midges (small blood-sucking flies in the family Ceratopogonidae) can transmit viruses, protozoans, and nematodes (Mullen and Murphree 2019). Bluetongue and epizootic hemorrhagic disease virus, which are spread by midges, result in infectious and sometimes fatal diseases of wild ungulates, particularly white-tailed deer (Odocoileus virginianus), and also may infect domestic ruminants such as sheep (Ovis aries) (Maclachlan et al. 2019). These diseases also may be spread horizontally (i.e., from one individual to another), without the need of a vector (Maclachlan et al. 2019). West Nile Virus, transmitted by mosquitos to birds, has had a particularly negative effect on birds in the crow family (Corvidae) (Kilpatrick et al. 2007). One of the areas of important future research with respect to these issues is how relationships of insects as disease vectors to domestic and wild animals are changing in the context of climate change and in relation to grassland condition.
6 Ecosystem Threats
6.1 Habitat Conversion
The global intensification of agriculture in recent decades has led to significant grassland conversion to cropland (see Chaps. 3, 5), with associated decreases of grassland bird (−20.8%) and shrubland bird declines (−16.5%) (Stanton et al. 2018). Insect populations also are responsive to changes in micro- and macrohabitats, including fragmentation, ecological disruption, and chemical pollution (Kremen et al. 1993) and all these issues can be associated with land conversion. Chen et al. (1995) documented changes in air temperature, soil temperature, relative humidity, short-wave radiation, and wind speed along the edge of a fragmented area. Fragmentation also may genetically isolate populations, making small populations more prone to extinction (Stacey and Taper 1992). As grasslands are modified, converted, and fragmented, all of these issues must be considered in the context of conservation of insects and wildlife.
6.2 Insecticides
The effect of insecticides on grassland insect populations may be significant, but effects are less well understood for insect species that are not pests. Chemicals used to control agricultural pest insects are not always specific in their effects and can affect non-target organisms both in terms of insects, but also with respect to earthworms (Lumbricus spp.), birds, and mammals (Sánchez-Bayo 2012). Even “biorational” insecticides (insecticides composed of natural products, including animals, plants, microbes, and minerals, or their derivates), which are considered less toxic than conventional chemical insecticides, can have unintended consequences on non-target insects (Haddi et al. 2020).
Insecticides also can have indirect effects on the wildlife that use insects as food. Swainson’s hawks are particularly susceptible to insecticides because they forage for large numbers of insects during insect outbreaks. Their susceptibility was discovered first relative to the use of organochlorides, but they are also vulnerable to other pesticides (Shaffer et al. 2019). Similarly, carbaryl and carbofuran, two insecticides used to control agricultural pests in corn and alfalfa fields, were found to decrease reproductive success of Burrowing Owls (Athene cunicularia hypugaea) when sprayed in proximity (50–400 m) to their burrows (Shaffer et al. 2022).
6.3 Nonnative Species
Nonnative species, and particularly nonnative plants, may have large, yet undetected effects on grassland insects. Roadways and hiking trails are prime areas for the introduction of nonnative plant species, because they are often transported via humans or horses (Larson 2002; Graves and Shapiro 2003). Some examples of nonnative plant species in western grasslands include cheatgrass (Bromus tectorum), Dalmatian toadflax (Linaria dalmatica), spotted knapweed (Centaurea maculosa), Canada thistle (Cirsium arvense), ox-eye daisy (Chrysanthemum leucanthemum), houndstongue (Cynoglossum officinale), and leafy spurge (Euphorbia esula). These species have indirect effects on the insect community by changing the amount and relative proportions of plant biomass and soil nutrients available to insects (Ehrenfeld 2003). This may benefit some insect species by providing additional nectar, food, or host plants (Graves and Shapiro 2003), but others may be negatively affected because their preferred nectar, food, or host plant species are out-competed by nonnatives (Levine et al. 2003).
In addition to nonnative plants, native insects may be affected by nonnative insects that can compete for habitat and resources. As noted previously, Apis melifera, the honey bee, is a nonnative species that may be having impacts on native bees in grasslands. In a meta-analysis of invasive species effects on rare and endangered insects worldwide, Wagner and Van Driesche (2010) found that invasive plants, ants, and vertebrate grazers and predators posed major threats to native insect biodiversity. The ecological effects of nonnative insects have only recently been assessed at a broader taxonomic and geographic scale (Kenis et al. 2009; Garnas et al. 2016) and this area of research is ripe for additional work as it relates to grassland conservation.
6.4 Climate Change
Climate change models predict warmer temperatures in U.S. rangelands with reduced snowpack and drier conditions in the Northwest, drier conditions in the southern Great Plains and southwest, and wetter conditions in the Great Plains (Briske et al. 2005). A recent model of overall productivity in North American grasslands predicts both earlier spring emergence and delayed autumn senescence of vegetation with climate change, resulting in increased grassland productivity despite some drought in summer seasons (Hufkens et al. 2016). However, there are a variety of interconnected relationships within rangelands that need to be considered. For example, issues of wind and water erosion associated with climate change could have serious implications for both rangeland health and human health (Edwards et al. 2019). Increasing variability of precipitation, woody plant encroachment, heat stress, and threat of drought will influence the future of how rangelands are managed in the context of climate change (Holechek et al. 2020).
Many insect species may be especially responsive to the predicted temperature and precipitation alterations of climate change because of their specialized habitat requirements, potentially making them sensitive indicators for altered ecosystems. Climate change may affect insects that have more specialized host plants more severely than those that can use a broad number of host plants. Within an individual plant species, responses to changes in precipitation, temperature, and carbon dioxide also could affect a grassland’s ability to support insect communities. Wenninger and Inouye (2008), in studying sagebrush steppe habitat in the eastern Snake River Plain, found that plants that are less water stressed harbor a greater diversity and abundance of insects. Insect diversity and abundance were positively correlated with both plant diversity and irrigation early in the summer, but by the end of the summer, insect distributions were more strongly influenced by irrigation treatment. In addition to the “filters” that the plant community type and plant physiological condition can have on insect distribution and abundance patterns, responses to climatic variation are also influenced by changes in predators and parasites in the system.
Climate change may be detrimental to some insect species that have narrow niches (i.e., they exist within a narrow set of environmental conditions) and advantageous to others that have broader niches (i.e., more generalist tendencies). Although some insects may respond quickly to climatic changes, their host plants and other members of the ecological community may not necessarily respond at the same rate. So, there is the potential for asynchrony in responses within the ecological community. Asynchrony can be detrimental, for example, if a plant blooms earlier but its pollinator comes out at the same time as in previous years (Maglianesi et al. 2020). The effects could then be manifested at higher levels of the food chain and affect grassland wildlife if plants produce fewer seeds for granivores or less biomass for herbivores. Given that plants and insects develop on a schedule of “degree days” (Sridhar and Reddy 2013), issues of asynchrony could potentially constitute a larger threat in communities where native vegetation has been replaced by introduced species.
Across all insect groups, butterflies have been most extensively studied in the context of climate change and may provide insights on the broader response of the insect community. Many butterfly species are associated with specific grassland types and, as such, butterfly communities have shown changes in distribution and abundance in the context of drought in Rocky Mountain grasslands (Debinski et al. 2013). Butterflies have been shown to exhibit rapid responses to climate change at a global level (Parmesan et al. 1999), and concern has recently been expressed about declines in butterfly species across the warming and drying western U.S. (Forister et al. 2021). Effects of a changing rangeland environment will be manifested in the productivity of the host plants and nectar resources used by many insects, which will impact insect survival and reproductive success. Whereas grasslands in the Rocky Mountains may be more susceptible to summer drought conditions due to reduced snowpack under climate change (Pederson et al. 2011, 2013), tallgrass prairie in Midwestern states may be more susceptible to spring flooding (Wuebbles and Hayhoe 2004). Both stressors will affect insect populations and communities, and this is another area where future research in needed.
7 Conservation and Management Actions
The conservation and management of insects in grasslands is still in its infancy compared to other wildlife species described within this volume. Due to high insect diversity and the lack of understanding of their ecosystem roles, only a small number of species have been well-studied in the context of management. Usually these are pest species, rare species, species of conservation concern, or threatened or endangered species, because designating a species in such a category justifies increased research funding for that species. Grasshoppers, due to their potential for creating large defoliation impacts in grasslands and crop fields, have been extensively studied because of apparent damage to crops. However, the benefits of insects are less easily observed. The American burying beetle and monarch butterfly are examples of species of concern that have been well-studied with respect to conservation and management. Bees, butterflies, ants, and, to a lesser degree, beetles, are some of the major groups of insects that have been evaluated for community responses to grassland management due to their importance in conservation education, pollination services, ecosystem engineering, and nutrient cycling respectively. The plethora of other insects are most often surveyed via sweep net and, due to the time-consuming nature of identifying them to species, are often summarized in terms of total biomass or abundance and diversity measure at the family rather than genus or species level, resulting in knowledge gaps in understanding of important relationships that may be occurring at the genus or species level.
Grassland restoration is one of the most effective methods of accomplishing conservation and management for grassland insects because high quality habitat is often a limiting resource for rare grassland insects. However, there are several important caveats to keep in mind when generalizing approaches to grassland restoration: (1) the effects of one type of management cannot be expected to result in the same vegetation response for all grasslands, (2) the effects of grazing, fire, and the combination thereof can vary based upon intensity, duration, timing, location, and year-to-year weather variation, (3) the historical management or use of a piece of land may have long-lasting legacy effects that influence the way that the land can and will respond to management, and (4) some grasslands are much easier to restore or reconstruct than others. Limitations of seed sources or precipitation make restoration and reconstruction of dry western grasslands much more difficult than in Midwestern grasslands. In understanding how insects respond to management, scientists and managers can be informed by experimental studies if sampling sites are well replicated and include experimental and control plots at a landscape scale studied over multiple years. Such experiments can provide guidance in developing an “adaptive management” approach so that prescriptions can be made for grasslands with specific locations, soil types, climate, and land management histories.
Just as grasslands can be managed for conservation, insect species also can be captively reared and reintroduced, and grasslands can be managed specifically for certain insect species. The regal fritillary butterfly was reintroduced to a restored tallgrass prairie in Iowa (Shepherd and Debinski 2005), and some insects are being reared by zoos in conservation efforts. Similarly, grassland habitats, both large and small, in many parts of the U.S. are being managed to improve habitat for monarch butterflies and pollinators. Public awareness has increased in recent decades regarding the value of creating pollinator habitat within grasslands, along roadways, on the edges of row crops and riparian areas, as well as within urban areas. However, conservation of habitat for insects has, to this point, primarily focused on pollinators, endangered species, and to a lesser extent biological control of pest insects. In the future, insect conservation could be expanded to focus on a broader suite of insect taxa, especially those conducive to engaging volunteers, school children, and/or citizen scientists.
8 Research and Management Needs
Future research linking insects, wildlife, and rangelands should focus on expanding our knowledge of how insects respond to both naturally occurring and human-induced ecological disturbances to grassland ecosystems including topics such as (1) the linkage between insect life history and responses to fire, and a better understanding of similarities and differences in responses between tallgrass prairie, shortgrass prairie, and Great Basin ecoregions, (2) the potential for differential responses of insects to climate change relative to their niche breadth, (3) the potential for asynchronous responses between insects and plants in the context of climate change, and (4) a broader understanding of the full taxonomic suite of insect population responses to chemicals used in grassland and agricultural management. Understanding the physiological responses, as well as the population, community, and ecosystem-level of responses of insects to fire, grazing, climate change, and chemical management, will allow future rangeland professionals to better predict and understand the complex roles that insects play in rangeland ecosystems and how changes in their distribution and abundance will affect associated wildlife species.
9 Summary
Insects have high reproductive rates and short life spans and can evolve and adapt quickly to abiotic and biotic changes in grasslands. Insects may be much smaller than their mammalian and avian counterparts at an individual level, but the total biomass they contribute to an ecosystem warrants notice, and the ecosystem services they provide are significant and critical. Insects are key intermediate components of the rangeland food web. Although the average grassland visitor may be more likely to stop and view the large mammals or birds in a grassland than the ants, beetles, bees, or grasshoppers, it is important to recognize that these “little creatures who run the word” (Wilson 1997) profoundly affect grassland wildlife populations, both directly and indirectly. As such, attention to insect conservation and the effects of management on insect distribution and abundance patterns is an essential component of holistic rangeland management.
References
Agnarsson I, Kuntner M (2007) Taxonomy in a changing world: seeking solutions for a science in crisis. Syst Biol 56:531–539
Atkinson TC, Briffa KR, Coope G (1987) Seasonal temperatures in Britain during the past 22,000 years, reconstructed using beetle remains. Nature 325:587–592
Auckland JN, Debinski DM, Clark WR (2004) Survival, movement, and resource use of the butterfly Parnassius clodius. Ecol Entomol 29:139–149
Baum KA, Wallen KE (2011) Potential bias in pan trapping as a function of floral abundance. J Kansas Entomol Soc 84:155–159
Belovsky G, Slade J (2000) Insect herbivory accelerates nutrient cycling and increases plant production. Proc Natl Acad Sci 97:14412–14417
Belsky J, Joshi NK (2018) Assessing role of major drivers in recent decline of monarch butterfly population in North America. Front Environ Sci 6
Borchers DL, Buckland ST, Zucchini W (2004) Estimating animal abundance: closed populations. Springer London Limited, London. https://doi.org/10.1007/978-1-4471-3708-5
Botzler RG, Brown RN (2014) Foundations of wildlife diseases, 1st edn. University of California Press, Berkerley. https://doi.org/10.1525/j.ctt7zw054
Briske DD, Fuhlendorf SD, Smeins FE (2005) State-and-transition models, thresholds, and rangeland health: a synthesis of ecological concepts and perspectives. Rangel Ecol Manage 58:1–10
Brittain C, Williams N, Kremen C et al (2013) Synergistic effects of non-Apis bees and honey bees for pollination services. Proc Royal Soc B Biol Sci 280:20122767
Buckland ST, Rexstad EA, Marques TA et al (2015) Distance sampling. Springer International Publishing AG, Cham
Butler JL, Parker MS, Murphy JT (2006) Efficacy of flea beetle control of Leafy Spurge in Montana and South Dakota. Rangel Ecol Manage 59:453–461
Cameron E (1913) Notes on Swainson’s Hawk (Buteo swainsoni) in Montana. Auk 30:381–394
Capinera J, Capinera DDJ (2010) Insects and wildlife: arthropods and their relationships with wild vertebrate animals, 1st edn. Wiley, Hoboken
Castrale JS (1982) Effects of two sagebrush control methods on nongame birds. J Wildlife Manage 945–952
Chen J, Franklin JF, Spies TA (1995) Growing-season microclimatic gradients from clearcut edges into old-growth Douglas-fir forests. Ecol Appl 5:74–86
Cook WM, Holt RD, Yao J (2001) Spatial variability in oviposition damage by periodical cicadas in a fragmented landscape. Oecologia 127:51–61
Davies KW, Bates JD, Boyd CS et al (2014) Is fire exclusion in mountain big sagebrush communities prudent? Soil nutrient, plant diversity and arthropod response to burning. Int J Wildland Fire 23:417–424
De Bruyn L, Thys S, Scheirs J et al (2001) Effects of vegetation and soil on species diversity of soil dwelling Diptera in a heathland ecosystem. J Insect Conserv 5:87–97
De Jong GD, Chadwick JW (1999) Decomposition and arthropod succession on exposed rabbit carrion during summer at high altitudes in Colorado, USA. J Med Entomol 36:833–845
Debano SJ (2006) Effects of livestock grazing on aboveground insect communities in semi-arid grasslands of southeastern Arizona. Biodivers Conserv 15:2547
Debinski DM, Babbit AM (1997) Butterfly species in native prairie and restored prairie. Prairie Naturalist 29:219–228
Debinski DM, Moranz RA, Delaney JT et al (2011) A cross-taxonomic comparison of insect responses to grassland management and land-use legacies. Ecosphere 2:art131
Debinski DM, Caruthers JC, Cook D et al (2013) Gradient-based habitat affinities predict species vulnerability to drought. Ecology 94:1036–1045
Delaney JT, Jokela KJ, Debinski DM (2015) Seasonal succession of pollinator floral resources in four types of grasslands. Ecosphere 6:1–14
Devillers J (2002) The ecological importance of honey bees and their relevance to ecotoxicology. Honeybees: estimating the environmental impact of chemicals. Taylor & Francis, London, pp 1–11
DiTomaso JM, Brooks ML, Allen EB et al (2006) Control of invasive weeds with prescribed burning. Weed Technol 20:535–548
Edwards BL, Webb NP, Brown DP et al (2019) Climate change impacts on wind and water erosion on US rangelands. J Soil Water Conserv 74:405–418
Ehrenfeld JG (2003) Effects of exotic plant invasions on soil nutrient cycling processes. Ecosystems 6:503–523
Ehrlich PR, Dobkin DS, Wheye D (1988) The birder’s handbook. Simon and Schuster, New York
Eldridge BF, Edman JD, Edman JD (2000) Medical entomology: a textbook on public health and veterinary problems caused by arthropods. Springer, The Netherlands, Dordrecht. https://doi.org/10.1007/978-94-011-6472-6
Ellingwood DD, Pekins PJ, Jones H et al (2020) Evaluating moose Alces alces population response to infestation level of winter ticks Dermacentor albipictus. Wildlife Biol
Erhardt A, Thomas J (1991) Lepidoptera as indicators of change in the semi-natural grasslands of lowland and upland Europe. Conserv Insects Habitats 112:213–236
Forister M, Halsch C, Nice C et al (2021) Fewer butterflies seen by community scientists across the warming and drying landscapes of the American West. Science 371:1042–1045
Fuhlendorf SD, Engle DM, Kerby J et al (2009) Pyric herbivory: rewilding landscapes through the recoupling of fire and grazing. Conserv Biol 23:588–598
Garnas JR, Auger-Rozenberg M-A, Roques A et al (2016) Complex patterns of global spread in invasive insects: eco-evolutionary and management consequences. Biol Invasions 18:935–952
Goldas CdS, Podgaiski LR, da Silva CVC et al (2021) Burning for grassland pollination: recently burned patches promote plant flowering and insect pollinators. Austral Ecol n/a
Goosey HB, Smith JT, O’Neill KM et al (2019) Ground-dwelling arthropod community response to livestock grazing: implications for avian conservation. Environ Entomol 48:856–866
Goulson D (2019) The insect apocalypse, and why it matters. Curr Biol 29:R967–R971
Graves SD, Shapiro AM (2003) Exotics as host plants of the California butterfly fauna. Biol Cons 110:413–433
Haddi K, Turchen LM, Viteri Jumbo LO et al (2020) Rethinking biorational insecticides for pest management: unintended effects and consequences. Pest Manag Sci 76:2286–2293
Hallmann CA, Sorg M, Jongejans E et al (2017) More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS ONE 12:e0185809
Hendrix SD, Kyhl FJ (2000) Population size and reproduction in Phlox pilosa. Conserv Biol 14:304–313
Hess JE, Beck JL (2014) Forb, insect, and soil response to burning and mowing Wyoming big sagebrush in greater sage-grouse breeding habitat. Environ Manage 53:813–822
Higgins KF, Kruse; AD, Piehl. JL (1987) Effects of fire in the Northern Great Plains. South Dakota State University Extension Circulars
Holechek JL, Geli HME, Cibils AF et al (2020) Climate change, rangelands, and sustainability of ranching in the Western United States. Sustainability 12:4942
Hufkens K, Keenan TF, Flanagan LB et al (2016) Productivity of North American grasslands is increased under future climate scenarios despite rising aridity. Nat Clim Chang 6:710–714
IUCN (2022, 07/21/2022) Migratory monarch butterfly now endangered—IUCN red list. https://www.iucn.org/press-release/202207/migratory-monarch-butterfly-now-endangered-iucn-red-list
Kaspari M, Joern A (1993) Prey choice by three insectivorous grassland birds: reevaluating opportunism. Oikos 414–430
Kelly L, Debinski DM (1998) Relationship of host plant density to size and abundance of the regal fritillary Speyeria idalia drury (Nymphalidae). J Lepidopterists Soc 52:262–276
Kemp WP (1992) Temporal variation in rangeland grasshopper (Orthoptera: Acrididae) communities in the steppe region of Montana, USA. Can Entomol 124:437–450
Kenis M, Auger-Rozenberg M-A, Roques A et al (2009) Ecological effects of invasive alien insects. Biol Invasions 11:21–45
Kilpatrick AM, LaDeau SL, Marra PP (2007) Ecology of West Nile virus transmission and its impact on birds in the western hemisphere. Auk 124:1121–1136
Knutson H, Campbell JB (1976) Relationships of grasshoppers (Acrididae) to burning, grazing, and range sites of native tallgrass prairie in Kansas. In: Tall timbers conference on ecological animal control habitat management proceedings, Gainesville, FL, 1974. Tall Timbers Research Station, Tallahassee FL pp 107–120, BIBL 1P1/2
Korb JE, Johnson NC, Covington WW (2004) Slash pile burning effects on soil biotic and chemical properties and plant establishment: recommendations for amelioration. Restor Ecol 12:52–62
Kral KC, Limb RF, Harmon JP et al (2017) Arthropods and fire: previous research shaping future conservation. Rangel Ecol Manage 70:589–598
Kremen C, Colwell R, Erwin T et al (1993) Terrestrial arthropod assemblages: their use in conservation planning. Conserv Biol 796–808
Kremen C, Williams NM, Aizen MA et al (2007) Pollination and other ecosystem services produced by mobile organisms: a conceptual framework for the effects of land-use change. Ecol Lett 10:299–314
Kromp B (1989) Carabid beetle communities (Carabidae, coleoptera) in biologically and conventionally farmed agroecosystems. Agr Ecosyst Environ 27:241–251
Lambe CM (2018) What’s all the buzz about: analyzing the decision to list the Rusty Patched Bumblebee on the endangered species list. Vill Envtl LJ 29:129
Larson DL (2002) Native weeds and exotic plants: relationships to disturbance in mixed-grass prairie. Plant Ecol 169:317–333
Larson B, Kevan P, Inouye DW (2001) Flies and flowers: taxonomic diversity of anthophiles and pollinators. Can Entomol 133:439–465
LaSalle J, Gauld I (1993) Hymenoptera: their biodiversity, and their impact on the diversity of other organisms. Hymenoptera Biodiversity 1–26
Lee K, Wood T (1971) Physical and chemical effects on soils of some Australian termites, and their pedological significance. Pedobiologia
Levine JM, Vila M, Antonio CMD et al (2003) Mechanisms underlying the impacts of exotic plant invasions. Proc R Soc Lond B 270:775–781
Losey JE, Vaughan M (2006) The economic value of ecological services provided by insects. Bioscience 56:311–323
Lussenhop J (1976) Soil arthropod response to prairie burning. Ecology 57:88–98
Maclachlan NJ, Zientara S, Wilson WC et al (2019) Bluetongue and epizootic hemorrhagic disease viruses: recent developments with these globally re-emerging arboviral infections of ruminants. Curr Opin Virol 34:56–62
Maglianesi MA, Hanson P, Brenes E et al (2020) High levels of phenological asynchrony between specialized pollinators and plants with short flowering phases. Ecology 101:e03162
Magurran AE (1988) Ecological diversity and its measurement. Princeton University Press, Princeton, NJ
Malmqvist B (2002) Aquatic invertebrates in riverine landscapes. Freshw Biol 47:679–694
Matthews EG, Kitching RL (1984). Insect ecology, 2nd ed
McGranahan DA, Engle DM, Fuhlendorf SD et al (2012) An invasive cool-season grass complicates prescribed fire management in a native warm-season grassland. Nat Areas J 32:208–214
McKnight SJ (2021) Resident monarch populations on the rise in California: what does this mean for the western migratory population? https://www.xerces.org/blog/resident-monarch-populations-on-rise-in-california-what-does-this-mean-for-western-migratory. Accessed 1 May 2022
Miller JR, Morton LW, Engle DM et al (2012) Nature reserves as catalysts for landscape change. Front Ecol Environ 10:144–152
Mills LS (2012) Conservation of wildlife populations: demography, genetics, and management, 2, Aufl. edn. Wiley-Blackwell, Hoboken
Mittelbach GG (2018) Community ecology. Sinauer Associates, Sunderland, Massachusetts
Mooring MS, Samuel W (1998) The biological basis of grooming in moose: programmed versus stimulus-driven grooming. Anim Behav 56:1561–1570
Moranz RA, Debinski DM, McGranahan DA et al (2012) Untangling the effects of fire, grazing, and land-use legacies on grassland butterfly communities. Biodivers Conserv 21:2719–2746
Moranz RA, Debinski DM, Winkler L et al (2013) Effects of grassland management practices on ant functional groups in central North America. J Insect Conserv 17:699–713
Mullen GR, Murphree CS (2019a) Biting midges (Ceratopogonidae). In: Medical and veterinary entomology. Elsevier, pp 213–236
Newbold TS, Stapp P, Levensailor KE et al (2014) Community responses of arthropods to a range of traditional and manipulated grazing in shortgrass steppe. Environ Entomol 43:556–568
Nichols E, Spector S, Louzada J et al (2008) Ecological functions and ecosystem services provided by Scarabaeinae dung beetles. Biol Cons 141:1461–1474
O’Neill KM, Olson BE, Wallander R et al (2010) Effects of livestock grazing on grasshopper abundance on a native rangeland in Montana. Environ Entomol 39:775–786
Ollerton J, Winfree R, Tarrant S (2011) How many flowering plants are pollinated by animals? Oikos 120:321–326
Pairis M, Sundermann J, Wang H (2003) Fire, mowing and soil moisture levels have no significant effects on underground arthropod population and diversity. Tillers 4:33–37
Parmesan C, Ryrholm N, Stefanescu C et al (1999) Poleward shifts in geographical ranges of butterfly species associated with regional warming. Nature 399:579–583
Paterson J, Forcherio C, Larson B et al (1995) The effects of fescue toxicosis on beef cattle productivity. J Anim Sci 73:889–898
Pederson GT, Gray ST, Woodhouse CA et al (2011) The unusual nature of recent snowpack declines in the North American cordillera. Science 333:332–335
Pederson GT, Betancourt JL, McCabe GJ (2013) Regional patterns and proximal causes of the recent snowpack decline in the Rocky Mountains, U.S. Geophys Res Lett 40:1811–1816
Pelton EM, Schultz CB, Jepsen SJ et al (2019) Western Monarch population plummets: status, probable causes, and recommended conservation actions. Front Ecol Evol 7
Petschenka G, Agrawal AA (2015) Milkweed butterfly resistance to plant toxins is linked to sequestration, not coping with a toxic diet. Proc Royal Soc B Biol Sci 282:20151865
Porrini C, Ghini S, Girotti S et al (2002) 11 Use of honey bees as bioindicators of environmental pollution in Italy. In: Honey bees: estimating the environmental impact of chemicals, p 186
Pritchard ZA, Hendriksma HP, St Clair AL et al (2021) Do viruses from managed honey bees (Hymenoptera: Apidae) endanger wild bees in native prairies? Environ Entomol
Radho-Toly S, Majer JD, Yates C (2001) Impact of fire on leaf nutrients, arthropod fauna and herbivory of native and exotic eucalypts in Kings Park, Perth, Western Australia. Austral Ecol 26:500–506
Ries L, Debinski DM, Wieland ML (2001) Conservation value of roadside prairie restoration to butterfly communities. Conserv Biol 15:401–411
Rowland MM (2019) The effects of management practices on grassland birds—greater sage-grouse (Centrocercus urophasianus). In: Johnson DH, Igl LD, Shaffer JA, DeLong JP (eds) The effects of management practices on grassland birds, chap. B. U.S. Geological Survey Professional Paper 1842. US Geological Survey. https://doi.org/10.3133/pp1842B
Rudgers JA, Clay K (2007) Endophyte symbiosis with tall fescue: how strong are the impacts on communities and ecosystems? Fungal Biol Rev 21:107–124
Sánchez-Bayo F (2012) Insecticides mode of action in relation to their toxicity to non-target organisms. J Environ Anal Toxicol 4:S4-002
Saunders WC, Fausch KD (2007) Improved grazing management increases terrestrial invertebrate inputs that feed trout in Wyoming rangeland streams. Trans Am Fish Soc 136:1216–1230
Schlicht DW, Orwig TT (1998) The status of Iowa’s Lepidoptera. J Iowa Acad Sci 105:82–88
Schmitz OJ (2003) Top predator control of plant biodiversity and productivity in an old-field ecosystem. Ecol Lett 6:156–163
Shaffer JA, Igl LD, Johnson DH, Sondreal ML, Goldade CM, Rabie PA, Thiele JP, Euliss BR (2022) The effects of management practices on grassland birds—Burrowing Owl (Athene cunicularia hypugaea). US Geological Survey. https://pubs.er.usgs.gov/publication/pp1842P
Shaffer JA, Igl LD, Johnson DH et al (2019) The effects of management practices on grassland birds—Swainson’s Hawk (Buteo swainsoni). In: The effects of management practices on grassland birds, US Geological Survey, vol Professional Paper 1842. US Geological Survey. https://doi.org/10.3133/pp1842M
Shepherd S, Debinski DM (2005) Reintroduction of Regal Fritillary (Speyeria idalia) to a restored prairie. Ecol Restoration 23
Sikes DS (1994) Influences of ungulate carcasses on coleopteran communities in Yellowstone National Park. Montana State University-Bozeman, College of Agriculture, USA
Sikes DS, Raithel CJ (2002) A review of hypotheses of decline of the endangered American burying beetle (Silphidae: Nicrophorus americanus Olivier). J Insect Conserv 6:103–113
Spafford RD, Lortie CJ (2013) Sweeping beauty: is grassland arthropod community composition effectively estimated by sweep netting? Ecol Evol 3:3347–3358
Sridhar V, Reddy PVR (2013) Use of degree days and plant phenology: a reliable tool for predicting insect pest activity under climate change conditions. In: Climate-resilient horticulture: adaptation and mitigation strategies. Springer, pp 287–294
Stacey PB, Taper M (1992) Environmental variation and the persistence of small populations. Ecol Appl 2:18–29
Stanton RL, Morrissey CA, Clark RG (2018) Analysis of trends and agricultural drivers of farmland bird declines in North America: a review. Agr Ecosyst Environ 254:244–254
Steelman CD (1976) Effects of external and internal arthropod parasites on domestic livestock production. Annu Rev Entomol 21:155–178
Stoner KJ, Joern A (2004) Landscape vs. local habitat scale influences to insect communities from tallgrass prairie remnants. Ecol Appl 14:1306–1320
Swengel AB (2001) A literature review of insect responses to fire, compared to other conservation managements of open habitat. Biodivers Conserv 10:1141–1169
Taylor RL, Maxwell BD, Boik RJ (2006) Indirect effects of herbicides on bird food resources and beneficial arthropods. Agr Ecosyst Environ 116:157–164
Turchin P, Taylor A, Reeve J (1999) Dynamical role of predators in population cycles of a forest insect: an experimental test. Science 285:1068–1071
Turin H, Den Boer P (1988) Changes in the distribution of carabid beetles in The Netherlands since 1880. II. Isolation of habitats and long-term time trends in the occurence of carabid species with different powers of dispersal (Coleoptera, Carabidae). Biol Cons 44:179–200
USDA (2022) Insects and pollinators. U.S. Department of Agriculture. Available via https://www.nrcs.usda.gov/wps/portal/nrcs/main/national/plantsanimals/pollinate/. Accessed 1 May 2022
USFWS (2020) U.S. fish and wildlife service finds endangered species act listing for monarch butterfly warranted but precluded. Available via https://www.fws.gov/press-release/2020-12/endangered-species-act-listing-monarch-butterfly-warranted-precluded. Accessed 1 May 2022
USFWS (2022) Environmental conservation online system. U.S. Fish and Wildlife Service. https://ecos.fws.gov/ecp/. Accessed 1 May 2022
Vandenbosch R (2003) Fluctuations of Vanessa cardui butterfly abundance with El Nino and Pacific Decadal oscillation climatic variables. Glob Change Biol 9:785–790
Vogel JA, Koford RR, Debinski DM (2010) Direct and indirect responses of tallgrass prairie butterflies to prescribed burning. J Insect Conserv 14:663–677
Wagner DL, Van Driesche RG (2010) Threats posed to rare or endangered insects by invasions of nonnative species. Annu Rev Entomol 55:547–568
Waldbauer G (2003) What good are bugs? Insects in the web of life. Harvard University Press
Welti EA, Joern A (2018) Fire and grazing modulate the structure and resistance of plant–floral visitor networks in a tallgrass prairie. Oecologia 186:517–528
Welti EA, Qiu F, Tetreault HM et al (2019) Fire, grazing and climate shape plant–grasshopper interactions in a tallgrass prairie. Funct Ecol 33:735–745
Wenninger EJ, Inouye RS (2008) Insect community response to plant diversity and productivity in a sagebrush–steppe ecosystem. J Arid Environ 72:24–33
Wilsey B (2018) The biology of grasslands. Oxford University Press, Oxford, UK
Wilsey BJ, Chalcraft DR, Bowles CM et al (2005) Relationships among indices suggest that richness is an incomplete surrogate for grassland biodiversity. Ecology 86:1178–1184
Wilson EO (1997) Little creatures who run the word. NOVA PBS Airdate, 12 Aug 1997
Wuebbles DJ, Hayhoe K (2004) Climate change projections for the United States Midwest. Mitig Adapt Strat Global Chang 9:335–363
Xerces (2022) Nebraska bumble bee atlas. https://www.nebraskabumblebeeatlas.org/. Accessed 1 May 2022
Zaller J, Kerschbaumer G, Rizzoli R et al (2015) Monitoring arthropods in protected grasslands: comparing pitfall trapping, quadrat sampling and video monitoring. Web Ecol 15:15–23
Zattara EE, Aizen MA (2020) Worldwide occurrence records reflect a global decline in bee species richness. Available at SSRN 3669390
Zoladeski CA, Maycock PF (1990) Dynamics of the boreal forest in northwestern Ontario. American Midland Naturalist 289–300
Acknowledgements
Thanks to H. Goosey, J. Shaffer, and M. Vaughan for comments that improved and broadened the topics covered in this chapter.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this chapter
Cite this chapter
Debinski, D.M. (2023). Insects in Grassland Ecosystems. In: McNew, L.B., Dahlgren, D.K., Beck, J.L. (eds) Rangeland Wildlife Ecology and Conservation . Springer, Cham. https://doi.org/10.1007/978-3-031-34037-6_26
Download citation
DOI: https://doi.org/10.1007/978-3-031-34037-6_26
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-34036-9
Online ISBN: 978-3-031-34037-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)