Abstract
Maintaining hydrological connectivity is important for sustaining freshwater fish populations as the high habitat connectivity supports large-scale fish movements, enabling individuals to express their natural behaviours and spatial ecology. Northern pike Esox lucius is a freshwater apex predator that requires access to a wide range of functional habitats across its lifecycle, including spatially discrete foraging and spawning areas. Here, pike movement ecology was assessed using acoustic telemetry and stable isotope analysis in the River Bure wetland system, eastern England, comprising of the Bure mainstem, the River Ant and Thurne tributaries, plus laterally connected lentic habitats, and a system of dykes and ditches. Of 44 tagged pike, 30 were tracked for over 100 days, with the majority of detections being in the laterally connected lentic habitats and dykes and ditches, but with similar numbers of pike detected across all macrohabitats. The movement metrics of these pike indicated high individual variability, with total ranges to over 26 km, total movements to over 1182 km and mean daily movements to over 2.9 km. Pike in the Thurne tributary were more vagile than those in the Ant and Bure, and with larger Thurne pike also having relatively high proportions of large-bodied and highly vagile common bream Abramis brama in their diet, suggesting the pike movements were potentially related to bream movements. These results indicate the high individual variability in pike movements, which was facilitated here by their access to a wide range of connected macrohabitats due to high hydrological connectivity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Longitudinal and lateral hydrological connectivity is important for sustaining fish assemblage structure and diversity (Amoros et al., 2002; Shao et al. 2019). However, global trends in fluvial connectivity are contrary to this, with most rivers around the world no longer being free flowing (Grill et al. 2019; Belletti et al. 2020). This disrupted connectivity tends to be associated with impacts on fish dispersal, with barriers located throughout the aquatic system that can limit the movement of anadromous, catadromous, and potamodromous fishes (Fullerton et al. 2010). Conversely, the maintenance of natural flow regimes and an absence of anthropogenic barriers provides high habitat connectivity, facilitates fish dispersal processes, and supports complex trophic dynamics through increasing access of fishes to more diverse foraging habitats that alter inter- and intra-specific interactions, and also affects nutrient cycling and productivity (Thieme et al. 2023). In connected systems, the movement of organisms across different habitat types allows for a more integrated food web where energy and nutrients can flow more freely through various trophic levels (Power and Dietrich 2002).
High habitat connectivity enables individual fish to express their natural traits and behaviours, which can vary considerably between individuals (Amat-Trigo et al. 2024). This variability is expressed through differences in spatial behaviours where, for example, some individuals are relatively sedentary, characterised by having small home ranges, while others are more active and have much larger home ranges (Gutmann Roberts et al. 2019; Amat-Trigo et al. 2024). Individual variability in the trophic niche often manifests as generalist populations being composed of relatively specialised individuals whose niches are small subsets of the population niche (Araújo et al. 2011). Consistency in individual specialization has been argued to have important ecological, evolutionary, and conservation consequences, including reducing levels of intraspecific competition and potentially improving population resilience through the minimising competitive pressures (Vander Zanden et al. 2010).
Reproductive activities and trophic interactions within populations can also profoundly shape the spatial habitat use of fish populations (Speed et al. 2010), such as instigating seasonal migratory events for spawning (Winter et al. 2021a) and more regular movements for foraging (Nunn et al. 2010). While daily foraging movements can be relatively limited in distance, the extent of spawning migrations can vary widely, ranging from a few kilometres for potamodromous species to over 1000 km for diadromous species (Verhelst et al. 2018; van Puijenbroek et al. 2019). However, traits, such as sex, age, length size and behaviour, can substantially influence the distribution, behaviour and movements of individuals (Koed et al. 2006; Nyqvist et al. 2020). The interplay of these variables thus has important implications for the spatial ecology of individuals, populations and communities.
The Northern pike Esox lucius (‘pike’) is a freshwater apex predator with a Holarctic range, being encountered across Europe and North America, and is recognised as a keystone piscivore (Craig 2008). Their diet can be highly plastic, with the consumption of prey that includes macroinvertebrates as well as fish (Pedreschi et al. 2015). Pike prey size is typically limited by gape size, and there are ontogenetic shifts in their diet, with larger individuals being capable of consuming larger-bodied prey (Nilsson and Brönmark 2000; Nolan et al. 2019). Pike movements display considerable intra-population variation along a continuum of spatial behaviours, where some individuals remain confined to relatively small areas (less than 1 km of river length), while others move repeatedly over several km (Masters et al. 2002). This variation can influence gene flow as highly vagile individuals move relatively large distances in search of suitable spawning habitats and mates (Ouellet-Cauchon et al. 2014). Additionally, it may increase their exposure to heightened risks of both intra- and interspecific competition, which can further impact their population dynamics (Nyqvist et al. 2018). Consequently, some pike populations consist of individuals with annual home ranges of less than 2 km, while others have home ranges exceeding 14 km (Sandlund et al. 2016). While there is usually a positive relationship between home range size and body size, this does not necessarily show allometric scaling. For example, large pike in a chalk stream in Southern England moved less than smaller individuals when their body mass was taken into account (Rosten et al. 2016). Longer distance movements of pike tend to be for spawning, with populations showing discernible partitioning between foraging and spawning areas (Craig 1996), which often include tributaries, side channels and ditches (Nyqvist et al. 2018; Oele et al. 2019). In the Baltic Sea, resident pike spawn in brackish coastal waters, while there is an anadromous form that spawns in freshwater streams and wetlands (Lappalainen et al. 2008). High habitat connectivity is thus important in enabling pike to express individual specialisation and to maintain their access to suitable spawning areas that then serve as nursery grounds.
In this study, the aim was to describe the movements of pike in a protected and highly connected wetland system, the lower River Bure network in eastern England, recognized for its ecological importance and designated as a Ramsar site. This aim included assessment of the influence of spawning, connectivity, individual traits and trophic ecology on the individual variability in movements between individual pike. The study system was the lower River Bure network in eastern England, a highly connected, free-flow wetland system comprising the main River Bure, connected side channels and stillwater habitats (Fig. 1). The movements of pike were measured using acoustic telemetry, a key tool for assessing the movements of animals in aquatic environments (Brownscombe et al. 2022), and complemented with the ecological application of stable isotope analysis (SIA), which provides information on trophic relationships and diet (Layman et al. 2012; Costantini et al. 2018). The objectives were to assess the individual variability in the movement metrics of pike over a 3-year period and the influence of body size, spawning season, and individual trophic ecology on this. We posit that larger pike will move greater distances, have larger home ranges, and consume larger-bodied prey compared with smaller pike, and all pike will move greater distances during spawning versus non-spawning periods. The study then quantifies the use of different macrohabitats by pike and explores how this habitat use varies across the study area. In completing these objectives, an increased understanding of pike behaviour and habitat use in lowland river systems is generated, providing important information on the management of wetlands, including the maintenance of hydrological connectivity.
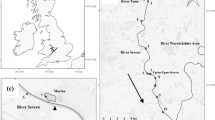
Map illustrating the study area within the Bure system, situated in Broad National Park, with red squares indicating the locations where acoustic receivers were deployed and the red triangle indicating the location of the station for environment variables (HOBO® Pendant; model MX2202, Onset Computer Corporation). The red arrows indicate the general flow
Methods
Study area
The study area was the northern region of the Broads National Park, Eastern England, comprising the lower River Bure and its tributaries of the Rivers Ant and Thurne (Fig. 1). This important wetland area has Ramsar status, which provides protection against habitat degradation and promotes sustainable land use practices (https://rsis.ramsar.org/ris/68). The system features more than 60 km of barrier-free main river, with a complex network of secondary slow-flow channels (< 2 m3/s) and numerous small shallow lakes (4 m depth), commonly known as “Broads” (Natural England, 2020). As a consequence, the system has high lateral and longitudinal connectivity, with the rivers intersected by a dense network of lateral broads and dyke systems (Fig. 1). The land use of the Broads primarily includes woodland and agriculture, with approximately 40% comprising of wet grassland grazed by livestock. As the lower Bure flows downstream, it transitions into semi-artificial grazing marsh and the water becomes more brackish owing to groundwater interaction with seawater (Winter et al. 2021a). The study area is influenced by tidal activity owing to its proximity to the sea, lack of barriers and the low-lying, relatively homogeneous topography of the landscape. The area is therefore prone to saline intrusion during large spring tides and storm surges in winter. The salt edge can be pushed more than 10 km inland during strong saline incursion events.
Fish capture, acoustic transmitter implantation and receiver network
Pike were sampled in the three main rivers (Bure, Ant and Thurne; Fig. 1). Rod and line angling was used to capture the fish as it was more effective in capturing large-bodied individuals compared with seine netting and electrofishing compromised by the increasing conductivity downstream. A total of 44 pike were captured; 16 were captured in November 2017, 27 in January 2018 and 1 additional pike was captured in September 2018 (Table S1). Before surgery, all fish were examined by the operator to assess any signs of stress and whether the length of the individual was appropriate for the size of the acoustic tag (all tagged fish were > 500 mm). Under general anaesthesia (Tricaine methanesulfonate, MS-222; approximately 0.04 g l−1), the pike were measured (fork length, nearest mm), sexed (external identification; Casselman 1974) and scale samples and pelvic fin biopsy taken (equivalent to approximately 1 mg dry weight), with the fin tissue frozen for storage. Each pike was then surgically implanted with an acoustic transmitter (Vemco V13; 69 kHz; 36 mm length × 13 mm diameter; mass in water: 6.0 g; random transmission interval approximately 90 s; estimated battery life: 1200 days; manufacturer: Innovasea, USA). The transmitters were inserted ventrally anterior to the pelvic fins, and incisions were closed using a single suture (2–0 absorbable monofilament; Ethicon Ltd, USA) and wound sealer (Orabase; ConvaTec Ltd, UK). During surgery, instruments were sterilised between fish though immersion in a 10% iodine based solution (Videne antiseptic solution; Ecolab Ltd, UK), followed by rinsing in 0.9% saline solution. During surgery, scales were removed from the incision site to facilitate the entry of the scalpel and sutures. Following completion of the surgical procedure, the fish were then transferred to tanks containing river water, held until normal body orientation and swimming behaviour resumed. After a visual inspection by the operator fish were released back to their approximate area of capture within 2 h of their tagging. During the same sampling periods, scales and fin biopsies were collected from 83 common bream (Abramis brama) and 47 roach (Rutilus rutilus) coming from the three different rivers. Additionally, macroinvertebrate samples were gathered from six distinct sites along the rivers that cover the main River Bure, Thurne and Ant, with sampling between 11 September and 3 October 2018 using a sweep net for stable isotope analysis (Table S2, Winter et al. 2021a,b,c). All surgical procedures were conducted by the same surgeon and in accordance with UK Home Office project license 70/8063 and after ethical review.
To track the movements of the tagged fish, a fixed network of 44 Vemco VR2W acoustic receivers was deployed in October 2017 and retrieved in summer 2020. These receivers were strategically placed to provide coverage of the length of the main rivers longitudinally (to enable long distance movements to be tracked). Some receivers were also positioned at the mouth of lateral connections (e.g., at the entrance of the broads from the main river) and inside channels considered to be important for pike foraging and spawning (based on extant knowledge of the authorship team) (Fig. 1). Receivers were attached to permanent structures, moored on navigation posts or suspended from floating objects, at approximately mid-water depth (1.0–1.5 m) to maximise detection efficiency during tidal changes in river levels. Detection data were downloaded every 4 months and batteries replaced annually. Movement data of the fish were collected from 2018 to 2020. All receivers remained operable throughout the study period and prior to any data analyses, the detection data were processed in actel R package (R version 4.2.3) to remove any false detections (e.g. transmitter code errors caused by code collisions) (Flávio and Baktoft 2021).
Stable isotope analysis and abiotic data collection
The fin biopsies of the pike, common bream and roach were individually used for stable isotope analysis (SIA). After drying at 60 °C for 24–36 h, the samples were ground and weighed to 1000 µg in tin capsules. These samples were then analysed for δ13C versus VPDB and δ15N versus At. Air (%) at the Cornell University Stable Isotope Laboratory, New York, USA, using a Thermo Delta V isotope ratio mass spectrometer interfaced with an NC2500 elemental analyser (CE Elantach Inc., USA). As C:N ratios were < 3.5, no lipid correction was applied. The same preparation and analyses were completed for the macro-invertebrate samples, a minimum of four replicates each capture site and date, with amphipods (Gammaridae) the most represented group, with other samples including killer shrimp Dikerogammarus villosus (Table S2, Winter et al. 2021c). In subsequent analyses, SI data of gammarids and killer shrimp were combined into a single group (‘Amphipods’), as differences in their values were not significant (t-test, p-value > 0.05).
Abiotic data were recorded by a stationary receiver (HOBO® Pendant; model MX2202, Onset Computer Corporation), maintained by the Environment Agency, and located in the main River Bure (52°38′56.5"N 1°34′03.5" E). Water temperature (± 0.5 °C), salinity and conductivity (us/cm) were recorded every 15 min throughout the entire study period.
Pike movement metrics
Of the 44 pike tagged, 41 were included in the calculation of movement metrics, with the remaining 3 individuals excluded owing to their lack of detections (Table S1). Data were downloaded from receivers using VUE software (version 2.7.0), and subsequent data manipulation was conducted using the Vtrack R package (Campbell et al. 2012), which enables the detection data of Vemco acoustic transmitters to be assimilated across all receivers and then analysed appropriately (Udyawer et al. 2018). To calculate river distances between detection points, the distance matrix was computed using the Field Calculator function in QGIS (version 3.28.8). The analyses completed here were a series of movement metrics as per Gutmann Roberts et al. (2019) (Table S1). Three indices were calculated to assess fish residency within the receiver array: the residency index, the linearity index and the residence array index (Table S1; Craig 2008; Acolas et al. 2017). The residence array index was included to address instances where a tagged fish might have been within the receiver array but remained undetected on a given day. The determination of total range (TR), expressed as the river distance (m) between the farthest receivers where an individual was detected, served as a proxy for home range. Additionally, total movement (TM), defined as the total distance covered by an individual, was computed by summing individual movements, regardless of their directionality (i.e. upstream, or downstream). In calculating mean daily movement (MDM), to avoid overestimates, only those occasions when the time between one observation and the next did not exceed 24 h were considered.
Kernel density estimation (KDE) was implemented to delineate the primary areas of pike spatial occupancy within the study area. This method involves computing the density of point features surrounding each output raster cell. A continuous, smoothly curved surface is then fitted over each point, with the surface exhibiting its highest value at the point’s location. This value gradually decreases as the distance from the point increases, eventually tapering off to zero at a distance equal to the specified search radius (Cantrell et al., 2018). KDE was performed by dividing the individuals according to their capture site (Bure, Ant and Thurne) and by dividing detections according to whether they were in the spawning period (1st Dec–30th Apr) or non-spawning period (1st May–31st Nov). These analyses were all conducted in ArcMap version 10.8.2. and generated heat maps that displayed the pike’s spatial occupancy probability. To assess variations in how the pike use the different macrohabitats across the study area, the receivers on which they were detected were then categorised according to whether the receiver was located in the main river (‘main’; as the Bure mainstem, and the Ant and Thurne tributaries), in ‘broads’ (the laterally connected, shallow, lentic habitats) or within the ditch and dyke systems (‘lateral’). For each river, the receiver detection data by macrohabitats were summed and converted to proportions (%). To see the differences in number of detections between habitats and wild populations, and isotopic signatures between the sites, we used a permutational univariate analysis of variance (PERANOVA), with Euclidean distance and 9999 permutations using the adonis2 function implemented in the R package vegan (Oksanen 2012).
Statistical analyses
The data collected in the first five days following each tagging event were excluded from the sample (in case the fish were demonstrating unusual post-surgical behaviours). Only pike that were detected for a period of at least 100 days (as days between the first and the last detection) were included (n = 30). This arbitrary threshold was chosen to focus on long-term movements and exclude shorter detections that could bias the results. The three main movement variables of each individual (TR, TM and MDM) were first tested at univariate level against total length (α = 0.05) and then included as response variables in different models and tested against the predictors (and their combinations) of the river of capture, spawning or non-spawning period (spawning period covered the pre-, spawning and the post-spawning period: 1 December to 30 April; non-spawning period: 1 May to 30 November), water temperature, salinity, fish identification (transmitter code), fork length and the proportion of common bream in diet (from stable isotope analyses; see below). With the response variables based on fish being detected for at least 100 days, the number of days of detection was not included as a predictor of any movement variable as the relationships were all non-significant (Pearson’s correlation coefficient, all r < 0.33, P > 0.05). Before model fitting, data exploration was undertaken following the protocol described in Ieno & Zurr (2015) involving examination for missing values, outliers in the response and explanatory variables, homogeneity and zero inflation in the response variable, collinearity between explanatory variables, the balance of categorical variables, and the nature of relationships between the response and explanatory variables.
To test differences in movement metrics between pike spawning and non-spawning periods, all movement metrics were subjected to analysis with ‘spawning’ designated as the predictor variable. The best fitting model for the total range of movement (‘TR’) was a linear mixed-effects model (LMM, ‘lme4’ R package), which was chosen due to the normally distributed nature of the TR data and to account for individual variability by including ‘FishID’ as a random effect (Table S3). To assess how pike utilized different macrohabitats during spawning versus non-spawning periods, a generalized linear mixed model (GLM) with generalized Poisson regression was employed. In this model, the response variable was the number of detections per receiver. The predictors included the macrohabitats of the main rivers (‘main’, comprising the Bure mainstem, Ant and Thurne tributaries) and the laterally connected habitats (‘lateral’, which included the broads, ditches, and dyke systems). For the analysis, the macrohabitats ‘broad’ and ‘lateral’ were combined as this enabled evaluation of the overall use of secondary connections versus the main river channels. As the total number of detections per receiver was not necessarily related to the number of fish detected (e.g. one individual could have been detected multiple times) the number of pike detected at each receiver in each period was included as a random effect. In addition, two other random effects were included in the model: the area of river the receiver was located (‘River’; as Bure, Ant and Thurne) and the antenna receiver identification number (‘ReceiverID’).
The relationship between pike length and movement patterns was analysed using a generalized linear mixed model (GLMM) with a gamma distribution. In this model, total movement (TM) was the response variable and fork length was the fixed factor. The GLMM was chosen to address the high variance and potential overdispersion in the TM data, providing a more accurate assessment of movement patterns. TM was used as other studies have observed that larger pike tend to move significantly more than small pike (day of detection and TM are autocorrelated) (Vehanen et al. 2006; Craig 2008; Sandlund et al. 2016). An additional interaction term between pike length and river of capture (as Bure, Thurne or Ant) was incorporated to accommodate potential disparities in movement tendencies across different river locations (Table S3). To then test the effect of abiotic variables on pike movement, a generalized additive model (GAM) was constructed using the 'mgcv' R package; a GAM was used owing to its ability to capture the nonlinear effects of temperature and salinity. Mean daily movement (MDM) was the response variable, given its sensitivity to fluctuations in environmental conditions, water temperature and salinity (daily mean) were included as predictor variables. Since the metrics are temporally repeated (across the season) to address individual variation, the fish unique identification code (FishID) was integrated as a random effect within the model structure (Table S3), with the number of knots set to 5 for all parameters to prevent overfitting. The model fitting process aimed to identify smooth trends that optimally captured the data while balancing goodness of fit and smoothness, with the final, best-fitting model identified by backward selection using AIC (ΔAIC ≤ 2).
Analyses of stable isotope data
To predict the dietary contribution of the putative prey species (common bream, roach, and macroinvertebrates) to pike diet, stable isotope mixing models were applied using the R package simmr (Parnell and Inger, 2016; Flávio and Baktoft 2021). To achieve this, we adopted a prior-free approach and applied trophic discrimination factors (TDFs) suggested by Post (2002), specifically 1.0 for δ13C and 3.4 for δ15N. Results of the mixing models are presented as a posteriori distribution for the proportion of each prey item in the individual diet of pike. The proportion of bream in pike diet was entered as the response variable in a GLMM to investigate potential associations with pike fork length. The predictor ‘river’ was also incorporated into the model to account for variations among the three primary rivers under study. This approach aimed to elucidate potential correlations between the dietary habits of pike and their size while considering differences within the study area.
Results
Fish length and detections
The mean fork length of the 41 pike analysed (31 female, 9 male, 1 undetermined) was 772 ± 122 mm (range 583–1143 mm), with females significantly larger than males (t-test, t = −5.111, p < 0.01) (Table S1). Detections of the tagged pike occurred across 933 days, with almost 3 million of individual detections, which were detected on individual receivers between 1117 and 521,771 times. Tracking periods were highly variable between individuals (3–931 days), with individuals being detected between 2 and 172 days of detection, with 30 fish having more than 100 days between their first and last detection (Table S1).
Movement metrics and macrohabitat use
Movement metrics varied between individuals (total range: 1487–26,099 m; total movement 8032–1,182,287 m; mean daily movement 46–2948 m; Table S1). For fish detected for at least 100 days, Thurne pike had the largest total ranges [mean ± 95% confidence interval (CI): 16,076 ± 5782 m], with Ant pike having higher total ranges (8141 ± 4951 m) than those in the Bure (5743 ± 1992 m). This was also reflected in the patterns of mean daily distance moved (Thurne: 1524 ± 383 m; Ant: 1261 ± 658 m; Bure: 717 ± 403 m). At a univariate level and across all fish, the movement metrics of total range, total distance moved and mean daily distance moved were not significantly related to fish length (linear regression, P > 0.05 in all cases, Fig. S1). The heat maps of pike spatial occupancy indicated most fish remained in the river where they were initially caught in both spawning and non-spawning periods (Fig. 2, Fig. S2). Individuals captured in the main River Bure were never detected in the other tributaries, while some individuals from the Ant and the Thurne were detected in the main Bure in both the spawning and non-spawning seasons (Fig. 2, Fig. S2). There were substantially more detections of pike on receivers located in the laterally connected macrohabitats than the main river (PERANOVA: p = 0.043), but the numbers of pike detected across these macrohabitats were similar (PERANOVA: p = 0.9) (Table S4). The GLM assessing the number of receiver detections across these macrohabitats revealed the influence of spawning season was not significant (P = 0.82), but the macrohabitat predictors were significant (‘Lateral’, P = 0.01; ‘Main’, P = 0.003), indicating the use of these different macrohabitats was important all year round (Table S5).
Heat maps of pike occupancy during the spawning (SW) and non-spawning period (NSW) in the river Bure system according to the location of tagging (Bure, Ant and Thurne), where the probability of occupancy ranges from absence (blue) through to low (green), medium (yellow) and high (red). Approximate release locations are noted with the black lines
Testing the relationships of the movement metrics in multivariate models revealed spawning season had a significant and positive effect on total range (LMM, P < 0.01; Table 2A), with the total range being on average 36% greater in the spawning season than on the non-spawning season. The fish identification interaction term was also significant, indicating that although movements were greater in spawning periods, this varied between individuals. The GLMs indicated pike length had a significant and positive effect on total movements (P = 0.03), where the interaction term between length and capture location was significant for the River Thurne (P = 0.02), indicating larger pike in this tributary moved more than those from the Bure and Ant (Table 1B). Mean daily distances moved were significantly and positively influenced by water temperature (GAM, P = 0.03; Table 1C), with the fish identification interaction term also being significant in the model, emphasising the individual variability in the dataset.
Pike diet composition by river
Mean δ13C of all pike was −27.55 ± 1.54% (−28.75% to −24.83%) and mean δ15N was 19.45 ± 1.05% (17.86% to 21.69%), with differences between sexes being not significant (t-test: δ13C, t = −0.26, p = 0.80; δ15N, t = −0.89, P = 0.39). Across all pike, there was a significantly higher value of δ13C as pike length increased (R2 = 0.16; F1,34 = 6.41, P = 0.02), but with no relationship between pike length and δ15N (R2 = 0.03; F1,34 = 0.90, P = 0.35) (Fig. S3). The common bream analysed for their stable isotope ratios were relatively large putative prey items versus roach (mean lengths ± 95% CI: bream 388 ± 13 mm, range 212–502 mm, n = 83); roach: 131 ± 11 mm, range 78–229 mm, n = 78). The common bream showed also a significantly higher δ13C ratio compared to roach (mean −28.78 ± 0.34 versus −29.92 ± 0.52%; t = 3.58, P < 0.01), but with no significant difference in δ15N (mean 17.05 ± 0.32 versus 17.29 ± 0.57%; t = −0.72, P = 0.48). The isotopic signatures of Bream and Roach showed no significant differences between the Bure and Thurne sites (PERANOVA: P = 0.9 and P = 0.18, respectively). However, both species exhibited distinct isotopic ratios in Thurne and Bure when compared with those in Ant (PERANOVA: P = 0.0015).
The diet composition predictions from stable isotope mixing models suggested that common bream was an important prey item for pike from the River Bure and Thurne (Fig. 3), where there was a significant relationship between increasing pike length and increased predicted bream dietary contribution (GLM, P < 0.02; Table 2). Conversely, pike in the River Ant were predicted to have relatively high dietary contributions of macroinvertebrates.
Discussion
Across the 30 pike used in movement analyses, there was high individual variability, with the pike moving total distances from 18 to 1182 km, with their mean daily movements being between 340 m and 3 km, with total ranges of 6–26 km. The individuals that moved more tended to be larger-bodied and were predicted to consume larger prey, with all movements tending to be greater during spawning versus non-spawning periods, with these results generally consistent with the predictions. The individual variability in pike movements and spatial behaviour observed here is also evident in other pike populations. For example, in the River Frome, southern England, pike revealed a continuum of spatial behaviours, where some individuals were always recorded in the same river reach of < 1 km length while others moved repeatedly over several km (Masters et al. 2002). In a Norwegian connected reservoir and river system, most pike had annual home ranges of less than 2 km, but with some individuals undertaking migrations of over 14 km (Sandlund et al. 2016). Indeed, individual variability in movements are also being apparent in a broad spectrum of species across aquatic and terrestrial environments (Shaw 2020). In entirety, these results emphasise the importance of maintaining high habitat connectivity in wetland systems to enable pike and other fishes to access a range of functional habitats over relatively large spatial areas.
Although some of the individual pike in this study were highly vagile, there was minimal mixing of pike between the three rivers. Pike tagged in the Bure were never detected in the Ant or Thurne, while pike tagged in the Ant and Thurne were only rarely detected in the main Bure. This might suggest a metapopulation structure, with fish in the Bure, Thurne and Ant comprising three distinct subpopulations with limited dispersal among them. This partitioning of pike into local groups with little ecological connectivity between them was also detected by Lukyanova et al. (2024) in both freshwater and brackish environments. Moreover, strong patterns of site fidelity in pike are often detected in telemetry studies, where movements away from core areas are limited, especially outside of the reproductive period (Miller et al. 2001; Kobler et al. 2008). For example, in the River Yser, Belgium, individually tagged pike demonstrated preferences for specific regions in the river in which they were detected most frequently (Pauwels et al. 2014) and in a German lake, translocated pike in the summer all returned to their main activity centre within 6 days, suggesting strong fidelity to these areas (Kobler et al. 2008).
The variability in movement metrics between individual pike was owing to some individuals moving between the different macrohabitats present in each river, with most detections occurring on receivers located in the laterally connected waters rather than the main river, but with similar fish numbers detected in all macrohabitats. This variability could have been influenced by variations in receiver detection efficiency between the different macrohabitats. Indeed, receiver efficiencies can be influenced by many biotic and abiotic factors, including temperature, precipitation, extent of vegetation and suspended sediments (Winter et al. 2021b). However, the receiver array used here was considered as highly reliable during the study period, with detection range very rarely falling below the width of the river (Winter et al. 2021b). Thus, the observed differences in pike detections and movements were considered as valid rather than an artefact of detection inefficiencies of the acoustic receivers. Moreover, high intra-population variability in movement ecology is a feature of many fish populations. In European barbel, the majority of individuals have relatively limited total ranges (< 10 km) but with a low proportion of individuals having much larger total ranges (Gutmann Roberts et al 2019; Amat Trigo et al., 2024). Juvenile Atlantic salmon Salmo salar predominantly have low mobility, adopting sedentary behaviour on most days, but with some individuals occasionally exhibiting bursts of high mobility, either by frequently moving within a confined area or moving between pools (Roy et al., 2013).
Highly connected systems are recognised as being important for providing the key functional habitats needed for all aspects of the pike lifecycle (Adolfsson 2020). Pike generally use wetland areas and back channels as spawning and nursery areas, which provide good habitats for larval production and recruitment compared with alternative habitats (Nilsson, Engstedt and Larsson, 2014). The use of these wetland areas by pike is, however, often limited to spring and early summer owing to their propensity of these areas to dry up later in summer, resulting in juveniles emigrating to more permanent waters as the waters recede, although some individuals will move earlier to either avoid cannibalism (Cucherousset et al. 2009; Nilsson et al. 2014) or owing to competitive displacement (Nyqvist et al. 2020). In the Bure wetland, however, spawning period had no influence on the number of receiver detections in the main river versus the laterally connected macro-habitats, with these lateral habitats important all year round. The fish assemblage of the ditch and dyke systems of the study area have previously been shown to be dominated by small numbers of pike, with their presence even recorded in the absence of prey fish species (other than occasional European eel Anguillia anguilla) (Townsend and Peirson 1988). Townsend and Peirson (1988) suggested that pike in these dyke systems would thus have diets heavily reliant on non-fish prey, including from terrestrial sources.
The suggestion that even adult pike diet can rely on non-fish prey by Townsend and Peirson (1988) has been supported by other studies where individual pike have been shown to be highly invertivorous (Beaudoin et al. 1999; Venturelli and Tonn 2005) including in pike of up to 60 cm fork length (Pedreschi et al. 2015). This trophic flexibility was also evident here, where stable isotope mixing models predicted that the diet of River Ant pike had relatively high proportions of macroinvertebrates when compared with those in the Bure and Thurne. In contrast, Bure and Thurne pike were predicted as having diets where common bream was an important – and large – prey item, with this dietary importance increasing as pike length increased (with common bream having higher values of δ13C compared with other aquatic resources, as well as being an abundant fish species (Winter et al. 2021a,c)). Ontogenetic dietary switches are common in pike through their development of larger gape sizes with increased body size, which facilitates their capture and handling of larger prey items (Bry et al. 1995; Nilsson and Brönmark 2000). In the lower River Severn, western Britain, the stable isotope ecology of pike of over 65 cm indicated that relatively large fish (> 30 cm) were important prey items, but with these prey items generally absent in the diet of smaller pike (Nolan et al. 2019). However, the putative prey samples collected for SIA were limited here to aquatic resources, with terrestrial resources not being considered as important to collect at the time of sampling. Accordingly, while we have confidence that the larger pike were consuming larger common bream as the reason for their higher values of δ13C (due to their high abundance in the system), we cannot discount that it could also related to some consumption of terrestrial prey items of relatively high δ13C.
The multivariate analyses indicated that the relationship between pike body length and total distance moved was significant and positive, with individuals in the Thurne also moving more than those in the Bure and Ant. In the River Frome, southern England, larger pike utilized 60% less of the river length, relative to their body size, compared with smaller individuals. This reduced movement is thought to result from larger pike expanding their home ranges, which increases spatial overlap between individuals, likely owing to high levels of intra-specific competition, with this posited as owing to the pike increasing their home range sizes – and thus the spatial overlap between individuals – owing to relatively high intra-specific competition (Jetz et al. 2004, Rosten et al. 2016). Such patterns were, however, not evident in our results, where the relationship between pike total range and body size was not significant. Instead, we posit that our larger pike generally moved more than smaller pike – especially in the River Thurne – as a response to movements of their prey species. This hypothesis is based on our results that indicated these larger pike not only moved significantly further than smaller pike but were also predicted to have diets comprising relatively large proportions of the highly vagile and abundant common bream. This hypothesis was, however, unable to be tested here owing to the use of low-resolution acoustic telemetry, which was unable to accurately quantify the movement relationships of the tagged pike with the movements of shoals of common bream. Indeed, a limitation of low resolution telemetry is that while it enables measurement of fish movements, it provides negligible information on the actual activity of those fish, coupled with it being unable to detect fish out of range of receivers, which is an issue in a species, such as pike that spend relatively long periods without moving far (Crossin et al. 2017; Jacoby and Piper 2023). Nevertheless, we speculate that predator–prey dynamics could have played a significant role in driving the movements of the larger tagged pike here, where they actively sought optimal foraging areas where large-bodied profitable prey, such as common bream, were more abundant (Goldbogen et al. 2015; Florko et al. 2023).
The study pike significantly increased their total ranges during the spawning period. Pike typically demonstrate a discernible partitioning between foraging and spawning areas, with fidelity expressed for specific spawning grounds year after year (Craig 1996), and their spawning migrations can be extensive, with some exceptional cases reaching up to 78 km (Carbine and Applegate, 1948 in Craig 1996), and with Baltic pike demonstrating anadromy during spawning (Lappalainen et al. 2008). Thus, the increased total range of Bure pike during spawning was likely owing to them making movements to specific spawning areas, although this could not be tested within our telemetry data owing to its low resolution that did not allow spawning areas to be reliably detected. Although there were limitations owing to having a single tracking station for environmental variables and therefore possibility of temperature fluctuations throughout the system, our analyses suggested these spawning movements were likely to be initiated by increasing water temperatures, with these warmer temperatures also playing a pivotal role in the spawning movements of other pike populations (Ovidio and Philippart, 2003). Although we argue our results provide important insights into the dynamics of pike movements in wetland systems, we acknowledge there are some limitations that necessitate some cautious interpretations. While the sample size provided valuable data, the relatively small number of analysed fish and the reliance on a single temperature monitoring station may have constrained our ability to fully capture the variability in pike behaviour and the influence of temperature on this. Additionally, the deployment of acoustic receivers, while generally efficient in detection, could have introduced variability in detection numbers across different habitats (given some areas had a relatively low receiver density), potentially influencing the spatial patterns in detections. The absence of terrestrial prey resources in our analysis also leaves room for further exploration of the trophic influences on pike movement. Consequently, while our study contributes to the understanding of pike ecology, it highlights the need for more extensive research to build on these findings and address the inherent challenges in studying complex aquatic systems.
In summary, there was high individual variability in the movements of pike in this wetland system, with larger pike moving more that potentially facilitated their ability to predate upon the highly vagile, abundant and large-bodied common bream. Although some pike were highly vagile, their movements were generally between different macrohabitats in each river, rather than being long distance movements between rivers. If the connectivity of these habitats were compromised owing to infrastructure developments, such as barriers, or declines in habitat quality, such as decreasing water levels and eutrophication (Ventura et al. 2023), pike might be compelled to cover greater distances in search of suitable functional habitats. This enforced dispersal could have a dual effect: on one hand, it might promote genetic flow between distinct pike metapopulations, potentially influencing genetic diversity (Ouellet-Cauchon et al. 2014), but could also result to increased energy expenditure, which might reduce overall fitness. This heightened energy demand could compromise the ability of individuals to effectively compete for spatial and food resources, potentially leading to increased risks of both interspecific and intraspecific competition (Bonte et al. 2012). Such extended movements might also expose fish to areas with varying resource availability (Cooke et al. 2022). Accordingly, these results highlight that preserving habitat connectivity is crucial for maintaining sustainable pike populations, as the connectivity ensures individuals can access diverse and profitable functional habitats throughout the year and express their full range of movement behaviours.
Data availability
Raw data used in the manuscript that are not already provided are available from the corresponding author upon reasonable request.
References
Acolas ML, Le Pichon C, Rochard E (2017) ‘Spring habitat use by stocked one year old European sturgeon Acipenser sturio in the freshwater-oligohaline area of the Gironde estuary. Estuarine Coastal Shelf Sci. https://doi.org/10.1016/j.ecss.2017.06.029
Adolfsson, O. (2020) Consequences on population dynamics following regained connectivity in pike (Esox lucius) spawning location. : https://urn.kb.se/resolve?urn=urn:nbn:se:lnu:diva-104213.
Amat-Trigo F et al (2024) Variability in the summer movements, habitat use and thermal biology of two fish species in a temperate river. Aquatic Sci. https://doi.org/10.1007/s00027-024-01073-y
Amoros C, Bornette G (2002) Connectivity and biocomplexity in waterbodies of riverine floodplains. Freshwater Biol. https://doi.org/10.1046/j.1365-2427.2002.00905.x
Araújo MS, Bolnick DI, Layman CA (2011) The ecological causes of individual specialisation. Ecol Letters. https://doi.org/10.1111/j.1461-0248.2011.01662.x
Beaudoin CP et al (1999) Individual specialization and trophic adaptability of northern pike (Esox lucius): an isotope and dietary analysis. Oecologia. https://doi.org/10.1007/s004420050871
Belletti B et al (2020) More than one million barriers fragment Europe s rivers. Nature. https://doi.org/10.1038/s41586-020-3005-2
Bonte D et al (2012) Costs of dispersal. Biol Rev. https://doi.org/10.1111/j.1469-185X.2011.00201.x
Brownscombe JW et al (2022) Application of telemetry and stable isotope analyses to inform the resource ecology and management of a marine fish. J Applied Ecol. https://doi.org/10.1111/1365-2664.14123
Bry C et al (1995) Early life characteristics of pike, Esox lucius, in rearing ponds: temporal survival pattern and ontogenetic diet shifts. J Fish Biol. https://doi.org/10.1111/j.1095-8649.1995.tb05949.x
Campbell HA et al (2012) V-Track: software for analysing and visualising animal movement from acoustic telemetry detections. Marine Freshwater Res. https://doi.org/10.1071/MF12194
Cantrell, D.L. et al. (2018) ‘The Use of Kernel Density Estimation With a Bio-Physical Model Provides a Method to Quantify Connectivity Among Salmon Farms: Spatial Planning and Management With Epidemiological Relevance , Frontiers in Veterinary Science. https://www.frontiersin.org/articles/https://doi.org/10.3389/fvets.2018.00269.
Casselman JM (1974) ‘External Sex Determination of Northern Pike, Esox lucius Linnaeus. Transact American Fisheries Society. https://doi.org/10.1577/1548-8659(1974)103%3c343:ESDONP%3e2.0.CO;2
Cooke SJ et al (2022) ‘The movement ecology of fishes. J Fish Biol. https://doi.org/10.1111/jfb.15153
Costantini ML et al (2018) ‘The role of alien fish (the centrarchid Micropterus salmoides) in lake food webs highlighted by stable isotope analysis ,. Freshwater Biol. https://doi.org/10.1111/fwb.13122
Craig JF (1996) Population dynamics, predation and role in the community. In: Craig JF (ed) Pike: Biology and exploitation. Dordrecht, Springer, Netherlands
Craig JF (2008) ‘A short review of pike ecology. Hydrobiologia. https://doi.org/10.1007/s10750-007-9262-3
Crossin GT et al (2017) Acoustic telemetry and fisheries management. Ecol Applicat. https://doi.org/10.1002/eap.1533
Cucherousset J et al (2009) Spatial behaviour of young-of-the-year northern pike (Esox lucius L) in a temporarily flooded nursery area. Ecol Freshwater Fish. https://doi.org/10.1111/j.1600-0633.2008.00349.x
Flávio H, Baktoft H (2021) actel: Standardised analysis of acoustic telemetry data from animals moving through receiver arrays. Met Ecol Evol. https://doi.org/10.1111/2041-210X.13503
Florko KRN et al (2023) The dynamic interaction between predator and prey drives mesopredator movement and foraging ecology. Bio. https://doi.org/10.1101/2023.04.27.538582
Fullerton AH et al (2010) ‘Hydrological connectivity for riverine fish: measurement challenges and research opportunities. Freshwater Biol. https://doi.org/10.1111/j.1365-2427.2010.02448.x
Goldbogen JA et al (2015) Prey density and distribution drive the three-dimensional foraging strategies of the largest filter feeder. Funct Ecol. https://doi.org/10.1111/1365-2435.12395
Grill G et al (2019) Mapping the world s free-flowing rivers. Nature. https://doi.org/10.1038/s41586-019-1111-9
Gutmann Roberts C, Hindes AM, Britton JR (2019) ‘Factors influencing individual movements and behaviours of invasive European barbel Barbus barbus in a regulated river. Hydrobiologia. https://doi.org/10.1007/s10750-018-3864-9
Home | Ramsar Sites Information Service. https://rsis.ramsar.org/.
Ieno, E.N. ‘A beginner s guide to data exploration and visualisation with R. : https://cir.nii.ac.jp/crid/1130848327913139328.
Jacoby DMP, Piper AT (2023) What acoustic telemetry can and cannot tell us about fish biology. J Fish Biol. https://doi.org/10.1111/jfb.15588
Kobler A, Klefoth T, Arlinghaus R (2008) ‘Site fidelity and seasonal changes in activity centre size of female pike Esox lucius in a small lake. J Fish Biol. https://doi.org/10.1111/j.1095-8649.2008.01952.x
Koed A et al (2006) Annual movement of adult pike (Esox lucius L.) in a lowland river. Ecol Freshwater Fish. https://doi.org/10.1111/j.1600-0633.2006.00136.x
Lappalainen A et al (2008) Reproduction of pike (Esox lucius) in reed belt shores of the SW coast of Finland, Baltic Sea: a new survey approach: Boreal Environment Research. Boreal Environ Res 13(4):370–380
Layman CA et al (2012) Applying stable isotopes to examine food-web structure: an overview of analytical tools. Biol Rev. https://doi.org/10.1111/j.1469-185X.2011.00208.x
Masters, J.E.G. et al. (2002) ‘Habitat utilisation by pike Esox lucius L. during winter floods in a southern English chalk river , in E.B. Thorstad, I.A. Fleming, and T.F. Næsje (eds) Aquatic Telemetry: Proceedings of the Fourth Conference on Fish Telemetry in Europe. Dordrecht, Springer, Netherlands.
Miller LM, Kallemeyn L, Senanan W (2001) Spawning-site and natal-site fidelity by northern pike in a large lake: mark-recapture and genetic evidence. Transaction American Fisher Societ. https://doi.org/10.1577/1548-8659(2001)130%3c0307:SSANSF%3e2.0.CO;2
Nilsson PA, Brönmark C (2000) ‘Prey vulnerability to a gape-size limited predator: behavioural and morphological impacts on northern pike piscivory. Oikos. https://doi.org/10.1034/j.1600-0706.2000.880310.x
Nilsson J, Engstedt O, Larsson P (2014) Wetlands for northern pike (Esox lucius L) recruitment in the Baltic Sea. Hydrobiologia. https://doi.org/10.1007/s10750-013-1656-9
Nolan ET, Gutmann Roberts C, Britton JR (2019) ‘Predicting the contributions of novel marine prey resources from angling and anadromy to the diet of a freshwater apex predator. Freshwater Biol. https://doi.org/10.1111/fwb.13326
Nunn AD et al (2010) ‘Seasonal and diel patterns in the migrations of fishes between a river and a floodplain tributary. Ecol Freshwater Fish. https://doi.org/10.1111/j.1600-0633.2009.00399.x
Nyqvist MJ et al (2018) Relationships between individual movement, trophic position and growth of juvenile pike (Esox lucius). Eco Freshwater Fish. https://doi.org/10.1111/eff.12355
Nyqvist MJ et al (2020) Dispersal strategies of juvenile pike (Esox lucius L.): Influences and consequences for body size, somatic growth and trophic position. Ecol Freshwater Fish. https://doi.org/10.1111/eff.12521
Oele DL et al (2019) Growth and recruitment dynamics of young-of-year northern pike: Implications for habitat conservation and management. Ecol Freshwater Fish. https://doi.org/10.1111/eff.12453
Oksanen, J. (2012) ‘Constrained Ordination: Tutorial with R and vegan.
Ouellet-Cauchon G et al (2014) Landscape variability explains spatial pattern of population structure of northern pike (Esox lucius) in a large fluvial system. Ecol Evolut. https://doi.org/10.1002/ece3.1121
Ovidio M, Philippart JC (2005) Long range seasonal movements of northern pike (Esox lucius L.) in the barbel zone of the River Ourthe. River Meuse basin, Belgium
Pauwels IS et al (2014) ‘Movement patterns of adult pike (Esox lucius L.) in a Belgian lowland river. Ecol Freshwater Fish. https://doi.org/10.1111/eff.12090
Pedreschi D et al (2015) ‘Trophic flexibility and opportunism in pike Esox lucius. J Fish Biol. https://doi.org/10.1111/jfb.12755
Power ME, Dietrich WE (2002) Food webs in river networks. Ecolog Res. https://doi.org/10.1046/j.1440-1703.2002.00503.x
Rosten CM, Gozlan RE, Lucas MC (2016) Allometric scaling of intraspecific space use. Biol Letters. https://doi.org/10.1098/rsbl.2015.0673
Roy L et al (2012) Individual variability in the movement behaviour of juvenile Atlantic salmon. Canadian J Fish Aquatic Sci [preprint]. https://doi.org/10.1139/cjfas-2012-0234
Sandlund OT, Museth J, Øistad S (2016) Migration, growth patterns, and diet of pike (Esox lucius) in a river reservoir and its inflowing river. Fisher Res. https://doi.org/10.1016/j.fishres.2015.08.010
Shao X et al (2019) River network connectivity and fish diversity. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2019.06.340
Shaw AK (2020) Causes and consequences of individual variation in animal movement. Move Ecol. https://doi.org/10.1186/s40462-020-0197-x
Speed CW et al (2010) Complexities of coastal shark movements and their implications for management. Marine Ecol Progres. https://doi.org/10.3354/meps08581
The Scaling of Animal Space Use | Science. https://www.science.org/doi/full/https://doi.org/10.1126/science.1102138.
Thieme M et al (2023) Measures to safeguard and restore river connectivity. Environment Rev [preprint]. https://doi.org/10.1139/er-2023-0019
Townsend CR, Peirson G (1988) Fish community structure in lowland drainage channels. J Fish Biol. https://doi.org/10.1111/j.1095-8649.1988.tb05362.x
Udyawer V et al (2018) A standardised framework for analysing animal detections from automated tracking arrays. Animal Biotelemet. https://doi.org/10.1186/s40317-018-0162-2
van Puijenbroek PJTM et al (2019) Species and river specific effects of river fragmentation on European anadromous fish species. River Res Application. https://doi.org/10.1002/rra.3386
Vander Zanden HB et al (2010) Individual specialists in a generalist population: results from a long-term stable isotope series. Biol Letter. https://doi.org/10.1098/rsbl.2010.0124
Vehanen T et al (2006) Patterns of movement of adult northern pike (Esox lucius L) in a regulated river. Ecol Freshwater Fish. https://doi.org/10.1111/j.1600-0633.2006.00151.x
Ventura M et al (2023) When climate change and overexploitation meet in volcanic lakes: the lesson from lake bracciano, rome’s strategic reservoir. Water. https://doi.org/10.3390/w15101959
Venturelli PA, Tonn WM (2005) Invertivory by northern pike (Esox lucius) structures communities of littoral macroinvertebrates in small boreal lakes. J North American Benthol Soci. https://doi.org/10.1899/04-128.1
Verhelst P et al (2018) Downstream migration of European eel (Anguilla anguilla L.) in an anthropogenically regulated freshwater system: Implications for management. Fisheries Res. https://doi.org/10.1016/j.fishres.2017.10.018
Winter ER et al (2021a) Acoustic telemetry reveals strong spatial preferences and mixing during successive spawning periods in a partially migratory common bream population. Aquatic Sci. https://doi.org/10.1007/s00027-021-00804-9
Winter ER et al (2021b) ‘Detection range and efficiency of acoustic telemetry receivers in a connected wetland system. Hydrobiologia. https://doi.org/10.1007/s10750-021-04556-3
Winter ER et al (2021c) ‘Dual-isotope isoscapes for predicting the scale of fish movements in lowland rivers. Ecosphere. https://doi.org/10.1002/ecs2.3456
Winter ER et al (2021d) ‘Movements of common bream Abramis brama in a highly connected, lowland wetland reveal sub-populations with diverse migration strategies. Freshwater Biol. https://doi.org/10.1111/fwb.13726
Acknowledgements
We thank the anglers for their assistance in pike sampling, especially the Pike Anglers Club of Great Britain (PAC) and Norwich and District Pike Club (NDPC). We also thank the Environment Agency for their logistical help in pike tagging
Funding
Bournemouth University,Environment Agency,TÜBİTAK BİDEB,2219 Program, EU LIFE + Nature and Biodiversity Programme, LIFE14NAT/UK/000054
Author information
Authors and Affiliations
Contributions
J.R.B., E.W., A.H., S.L., R.W. and J.L. conceived the study; J.R.B., E.W., A.H. and S.L. captured and tagged the fish; S.C., E.W., A.S.T. and S.A. analysed the data; S.C., J.R.B. and A.S.T. wrote the manuscript, and all authors edited the manuscript, and all authors agree to its submission.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cittadino, S., Tarkan, A.S., Aksu, S. et al. Individual variability in the movement ecology of Northern pike Esox lucius in a highly connected wetland system. Aquat Sci 86, 105 (2024). https://doi.org/10.1007/s00027-024-01124-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00027-024-01124-4