Abstract
Lenalidomide, bortezomib, and dexamethasone (RVd) have previously been established as standard-of-care induction therapy for newly diagnosed multiple myeloma (NDMM). More recently, randomized phase 3 data have demonstrated the benefit of the addition of daratumumab (Dara-RVd) to the RVd backbone in terms of improved both depth of response and long-term survival benefit as measured by progression-free survival (PFS). Our group has previously published on a historical cohort of 1000 NDMM patients uniformly treated with RVd induction with impressive both PFS and overall survival. Here, we present a comparative analysis of our RVd cohort with a recent cohort of 326 patients induced with Dara-RVd at our institution with intent to transplant. This analysis demonstrates the utility of this regimen in real-world clinical practice and provides additional insights into D-RVd performance in patient subsets often underrepresented in clinical trials, as well as the impact of daratumumab in maintenance for NDMM patients.
Similar content being viewed by others
Introduction
There have been significant improvements in clinical outcomes as measured by progression-free survival (PFS) and overall survival (OS) in myeloma patients due to rapid therapeutic development in the field. The combination of lenalidomide, bortezomib, and dexamethasone (RVd) was shown to be highly effective in newly diagnosed myeloma (NDMM) patients [1], leading to early adoption of RVd into our standard of care clinical practice. This experience has previously been published, reporting on 1000 consecutive NDMM patients treated with RVd followed by HDT/ASCT and risk-stratified and continuous maintenance therapy until progression with the added benefit of long-term follow-up reporting an impressive median PFS of 68.7 months and median OS of nearly 11 years for the entire cohort with a median follow up of 7.4 years [2, 3]. Though this analysis was retrospective, this is the largest database of NDMM patients treated with a uniform methodology to date, and is demonstrative of the efficacy of RVd in conjunction with ASCT and maintenance therapy, delivering durable remissions and survival outcomes [4].
The GRIFFIN trial is a randomized phase 2 trial comparing RVd versus RVd with daratumumab (Dara-RVd), a monoclonal anti-CD38 antibody, followed by HDT/ASCT and either lenalidomide or daratumumab and lenalidomide maintenance for two years in transplant-eligible NDMM. The study met its primary endpoint of 42% versus 32% stringent complete response (sCR) rate post-consolidation favoring the quadruplet with a trend towards survival benefit, again favoring the quadruplet arm [5]. The PERSEUS trial is the supporting registrational randomized phase 3 trial that evaluated D-RVd versus RVd among transplant-eligible NDMM patients with a 48-month PFS rate of 84.3% versus 67.7% for D-RVd versus RVd (HR 0.42; 95% CI 0.30–0.59; p < 0.001), and MRD negativity rate also favoring the quadruplet arm (75.2% versus 47.5%, p < 0.001). In sum, this randomized data is quite convincing and supports quadruplet induction therapy as the new standard of care [6].
Recognizing the potential benefit of daratumumab in induction, we modified our standard of care treatment to Dara-RVd for NDMM patients at our institution in 2018. Since that time, we have compiled a database of 326 consecutive transplant-eligible NDMM patients treated with D-RVd followed by HDT/ASCT and risk-stratified continuous maintenance therapy until progression. Here, we present both an analysis of the D-RVd dataset, as well as a real-world comparison of the largest cohort of patients consecutively treated with either D-RVd or RVd induction therapy reporting on response rates and long-term outcomes for both standard- and high-risk patients.
Methods
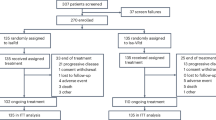
We identified 1000 consecutive NDMM patients treated with RVd between January 2007 and August 2016, and 326 NDMM patients treated with D-RVd induction therapy from April 2018 to August 2022 that were included in this analysis. Demographic and clinical characteristics and outcomes data were obtained from our institutional review board-approved myeloma database and by manual abstraction. Responses and progression were evaluated per International Myeloma Working Group (IMWG) Uniform Response Criteria. Genetic risk-stratification was done in accordance with the IMWG definition and defined by the presence of deletion (17p), translocation (4;14), and (14;16) by either fluorescence in-situ hybridization (FISH) on CD138 selected cells or by metaphase cytogenetics. Double hit was defined as the presence of two of the following abnormalities: +1q21, del(17p), and an IgH translocation involving chromosomes 4, 16, and 20.
International Staging System (ISS) and the revised ISS [7] were calculated for patients with available lab values of β2-microglobulin, albumin, lactate dehydrogenase (LDH) level, and genetic risk stratification at diagnosis. Response assessment and progression were evaluated per IMWG Uniform Response Criteria [8]. Further information on information regarding patient selection and dosing schedule for the RVd database has been previously published [2].
For the D-RVd dataset, all transplant-eligible NDMM patients referred to our center during the above timeframe were screened for a selected induction regimen. From the initial identification of 351 patients in the D-RVd dataset, three were removed as restaging data was not available, and 22 patients were removed because they had received more than one cycle of an alternative induction regimen prior to initiating D-RVd.
To ensure homogeneity and consistent group practice, all patients were discussed, and an algorithm for uniformity was followed consistently. Daratumumab was dosed at 1800 mg subcutaneously on days 1, 8, and 15 of a 21-day cycle. Lenalidomide was dosed at 25 mg/day on days 1–14 of a 21-day cycle. Bortezomib was given subcutaneously at 1.3 mg/m2 on days 1, 4, 8, and 11 of the 21-day cycle, and dexamethasone was dosed at 40 mg either once weekly or as tolerated. Dose adjustments were made based on the treating physician’s discretion and patient tolerability. All patients were treated with D-RVd for 4–6 cycles, pending the achievement of at least a partial response as defined by the IMWG response criteria. Our approach for stem cell mobilization and transplant approach has been previously described [2]. For maintenance, standard-risk patients received single-agent lenalidomide, while high-risk patients received a combination of PI and IMiD post-transplant. For all patients who opted for deferred transplant, the standard treatment approach was for those patients to undergo stem cell mobilization and collection upfront and then be initiated on maintenance therapy per physician discretion with the intent to proceed to transplant at first relapse.
Statistical analysis
SPSS package version 26 (Chicago, IL) was used for statistical analysis. Chi-square test and Fisher’s exact tests were used while comparing differences between categorical variables, and the non-parametric Mann–Whitney test was used for continuous variables. Survival projections of PFS and OS were estimated by the Kaplan–Meier method and compared by log-rank tests. The Cox proportional hazards model was used to assess predictors of PFS and OS. Only selected variables reaching statistical significance of p-value < 0.05 on univariate analysis were included in the multivariate analysis for both PFS and OS. PFS was calculated as the time from diagnosis of myeloma to disease progression or mortality from any cause. OS was defined as the time from diagnosis to the date of death or last follow-up.
Results
Patient characteristics
Patient characteristics for D-RVd versus RVd are listed in Table 1. The median age of patients was similar between the two groups, and there was no significant difference in sex distribution. Consistent with our catchment area, 41.7% of patients in the D-RVd cohort and 36.3% in the RVd arm are black. Of note, for D-RVd versus RVd, 13.8% versus 15.8% have high-risk disease, 16% versus 23.3% have ISS 3, and 4.6% versus 11.5% have RISS 3 disease. Among the D-RVd cohort, ISS data was available for 79.1% of patients, and RISS data was available for 75.4% of patients For the RVd and D-RVd cohort, respectively, 98.6% and 99.7% of patients underwent ASCT. In the D-RVd group, 311 (95%) patients underwent upfront ASCT compared to 75.1% in the RVd cohort.
Maintenance data for the D-RVd cohort is available for 83.4% of patients at the time of data cut-off. Of these 272 patients, 84.6% were started on lenalidomide maintenance. The remainder of patients on maintenance were on triplet maintenance regimens mostly due to high-risk disease. The most common triplet maintenance regimens used were KRd (6.6%), KPd (2.6%), DRd (2.2%), and RVd (1.5%). In the RVd cohort, 75.1% of patients received maintenance, with a vast majority of these patients (80%) receiving lenalidomide monotherapy and 14.2% of patients receiving a PI/IMID backbone.
Response rates
The post-induction overall response rate (ORR) is 99.6% in D-RVd versus 97.1% in RVd, with ≥VGPR rates of 86.5% versus 67.6%, respectively. Post-transplant ORR is 99.2% versus 98.6%, with ≥VGPR rates of 95.6% versus 86.8%, respectively. Post induction, the CR/sCR rate for D-RVd is 21.5% and 42.8% post-transplant, which is appreciably less than what was observed in the RVd cohort (35.9% and 67.5%). (Table 2) This discrepancy is likely explained by interference in the serum immunofixation assay by daratumumab, an IgG kappa monoclonal antibody migrating as an oligoclonal band. 318 patients (97.5%) underwent at least one stem cell collection attempt. Of these patients, six patients (1.8%) required a second stem cell collection attempt, and one of these patients failed to collect altogether despite two attempts and the use of cyclophosphamide mobilization with the second attempt. Of the patients who successfully collected, the median number of days of apheresis was 1.87 (range, 1–5), and the median number of CD34 cells collected was 9.35 million cells/kg (range, 0–20.46).
The median follow-up time for the RVd cohort was 90 months, and for the D-RVd cohort was 19.1 months from the time of diagnosis. Though the median follow-up for the Dara-RVd cohort is significantly shorter compared to the RVd cohort, a PFS benefit is demonstrated, favoring the quadruplets among both standard- and high-risk patients. The 2-year PFS D-RVd versus RVd is 93% versus 82% (p < 0.001), and the 2-year OS for D-RVd versus RVd is 94% versus 91% (p < 0.034). (Fig. 1) For standard-risk patients, the 2-year PFS for D-RVd versus RVd is 94% versus 84% (p < 0.001), and for high-risk patients, 83% versus 69% (p < 0.031). The 2-year OS for standard risk patients was 96% in D-RVd versus 93% in RVd (p < 0.001), and 94% versus 79% in HR patients (p = 0.06). (Fig. 2) OS estimates for HR patients also favored D-RVd, though this is more likely than PFS to be impacted by changes in treatment patterns over the past decade (Table 3). When looking at high-risk disease as classified by RISS stages 2 and 3 disease, we again see an early trend towards benefit with D-RVd induction versus RVd with a mPFS not reached versus 49.9 months, respectively (p < 0.001) (Fig. 3). In multivariate analysis for PFS, there is an impressive 72% reduction in the risk of progression or death for all patients, with the benefit clearly less pronounced in R-ISS 3 disease, and patients who were not on maintenance therapy (Table 4).
A Progression-free survival for standard-risk NDMM patients treated with D-RVd versus RVd. B Progression-free survival for high-risk NDMM patients treated with D-RVd versus RVd. C Overall survival for standard-risk NDMM patients treated with D-RVd versus RVd. D Overall survival for high-risk NDMM patients treated with D-RVd versus RVd.
When evaluating the impact of quadruplet induction in black patients compared with white patients, there is no statistically significant difference in PFS in either the D-RVd or RVd datasets. Moreover, the benefit of adding daratumumab is of similar magnitude between both black and white patients when adding daratumumab to the RVd backbone (Fig. 4).
When we specifically analyze the more recent and contemporaneous cohort of our RVd analysis, meaning the last 326 patients treated with RVd of the entire 1000 patient cohort, the HR for disease progression or death with D-RVd is 0.38 (95% CI 0.18–0.85; p < 0.017) (Fig. 5). Understanding the potential impact of the differing numbers of deferred ASCT in each cohort, we also examined the PFS of only patients who underwent upfront ASCT. Again, PFS favors the quadruplet with a median PFS of 65.8 months in the RVd arm and NR in the D-RVd with current follow-up (Fig. S1).
Discussion
The potential benefit of adding daratumumab to either induction and/or maintenance is an important question in our field. Daratumumab is an effective anti-myeloma therapy and is well tolerated and now even easier to administer in the subcutaneous formulation. The recently published PERSEUS study has established D-RVd as a standard of care induction therapy in NDMM, and this analysis can provide further information on this regimen in a real-world population with underrepresented subgroups, as well as additional insight into the role of daratumumab in the maintenance setting.
While no retrospective analysis can replicate the balance of a randomized clinical trial, in this comparison, patient characteristics were similar in terms of age, sex, and race between the RVd and D-RVd cohorts. Risk-stratified maintenance therapy was used in both patient populations, with a vast majority of standard-risk patients receiving lenalidomide monotherapy until progression, and high-risk patients receiving triplet maintenance therapy with a PI and IMID backbone. There was a higher percentage of patients in the D-RVd group with carfilzomib-based maintenance strategies when compared to RVd, but this accounted for a very small number of patients overall.
In terms of response rates, we saw improved depth of response favoring the D-RVd group with a higher ORR as well as higher ≥VGPR rates. The sCR/CR rates were lower in the D-RVd group, but again, this is almost certainly due to the daratumumab interference with the serum immunofixation assay. Unfortunately, MRD data was not routinely available yet post-transplant for all patients, though this analysis is ongoing. However, the depth of response benefit with quadruplets did translate to an early PFS benefit for all patients, and this benefit was seen for both the standard- and high-risk patient groups.
Beyond the GRIFFIN and PERSEUS trials, multiple randomized phase 3 studies have also shown the benefit of quadruplet induction regimens with the utilization of an anti-CD38 monoclonal antibody, either daratumumab or isatuximab, in combination with a PI and IMID backbone [9, 10]. The GMMG-HD7 trial was a randomized phase 3 trial comparing isatuximab and RVd versus RVd, and post-induction rates favored the quadruplet arm with a ≥VGPR rate of 77% versus 61% and improved MRD negativity rate of 50% versus 36% [10]. Several phase 2 trials have also investigated the use of quadruplet induction with daratumumab, as well as the use of the more potent proteasome inhibitor, carfilzomib, in lieu of bortezomib. However, given the increased toxicity seen with carfilzomib as compared to bortezomib and the small number of patients included in these trials, though the early data is quite impressive, we support the use of Dara-RVd for both standard and high-risk patients [11,12,13].
It is important to clarify the differences in the dosing schedule utilized in our analysis and in the GRIFFIN and PERSEUS trials. Induction was administered in 21-day cycles in contrast to 28-day cycles with weekly dosing of daratumumab, lenalidomide on days 1–14, and bortezomib twice weekly on days 1, 4, 8, and 11. Dexamethasone was dosed at our center at 40 mg weekly unless split dosing was required due to patient toxicity versus 20 mg on days 1, 2, 8, 9, 15, and 16. Furthermore, post-transplant, per our institutional protocol, we initiated maintenance therapy without the requirement of two additional cycles of consolidation, as was done in both the GRIFFIN and PERSEUS trials. We also did not use daratumumab during maintenance therapy as was done in the D-RVd arms in the GRIFFIN and PERSEUS trials and instead utilized the previously described risk-stratified maintenance approach per our standard institutional practice [14].
There are several limitations to this analysis, namely that it is a retrospective study and is a comparison of two sequential cohorts separated by time with varying availability of supportive care and novel treatment options. We tried to mitigate this potential difference with our subgroup analysis of the last 326 patients included in RVD 1000, which clearly shows the superiority of the quadruplet regimen. We do not have specific data on adverse events, quality of life, or dose reductions/holds during induction and/or maintenance therapy. Additionally, as some of the patients were induced in the community and then subsequently transplanted at our center, though we recommended our standard dosing of D-RVd, we do not have confirmation that this is how the regimen was administered.
Despite these limitations, this is the largest real-world database of transplant-eligible NDMM patients treated with RVd versus D-RVd, and importantly, the control arm (RVd) has historically mimicked the RVd arm from randomized trials providing further confidence to the validity of this data. Though we now have data on D-RVd from a large, randomized phase 3 trial, there are key differences between our datasets and the clinical trial experience that lend additional perspective on the treatment of NDMM. Importantly, both the RVd and the D-RVd datasets offer valuable insight into response and outcomes in black patients as they include a much larger number than seen in randomized prospective trials (36.3% in the RVd dataset and 41.7% in the D-RVd dataset). There was no statistically significant difference in PFS between black and white patients treated with either D-RVd or RVd. Moreover, black patients benefited from D-RVd induction with a similar magnitude of benefit as compared to RVd as their white counterparts, suggesting that if black patients are afforded the same access to care, they can experience the same outcomes.
Another unique aspect of our treatment algorithm as compared to GRIFFIN and PERSEUS is our risk-adapted maintenance approach. Though it is clear multiagent maintenance is necessary for some patients, it remains unclear which patients actually benefit from this more intensive approach, and thus we currently reserve this strategy for higher-risk patients. Despite this difference between our treatment approach and the randomized data, we found that our daratumumab arm has a similar estimated 4-year PFS rate compared to the PERSEUS trial. This raises the question about the necessity of daratumumab in both induction and maintenance for all patients. Our analysis suggests good outcomes with daratumumab in induction, but longer follow-up is needed to confirm which subsets of patients truly benefit from daratumumab in the maintenance setting as well. This question has previously been raised in the CASSIOPEIA trial, as patients who received daratumumab as induction did not seem to benefit from daratumumab as maintenance therapy and vice versa. Again, longer-term follow-up will be needed to evaluate the true benefit of the addition of daratumumab during maintenance.
Looking forward, we feel that these results, in conjunction with the GRIFFIN and PERSEUS data, as well as other randomized trials evaluating the addition of daratumumab to triplet induction regimens, support the use of Dara-RVd as the standard of care induction therapy for both standard- and high-risk NDMM patients, followed by ASCT and maintenance therapy. We acknowledge that for particular subsets of high-risk MM patients, such as double-hit disease, this strategy is likely suboptimal and supports the ongoing clinical investigation of the use of novel immune-based strategies, including earlier use of bispecific T-cell engagers and possibly CAR-T cell therapy for this patient group.
Conclusion
D-RVd is a highly effective induction regimen that can improve upon outcomes compared to a historical NDMM population treated with RVd in terms of depth of response and PFS benefit. In conjunction with randomized phase 3 data supporting D-RVd versus RVd as the standard of care induction, this analysis further supports D-RVd as the standard of care induction for both standard- and high-risk NDMM patients and provides confidence that these beneficial results can translate from the clinical trial setting to clinical practice.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Richardson PG, Jacobus SJ, Weller EA, Hassoun H, Lonial S, Raje NS, et al. Triplet therapy, transplantation, and maintenance until progression in myeloma. N Engl J Med. 2022;387(2):132–47. https://doi.org/10.1056/NEJMoa2204925
Joseph N, Kaufman J, Dhodapkar M, Hofmeister C, Almaula D, Heffner L, et al. Long-term follow-up results of lenalidomide, bortezomib, and dexamethasone induction therapy and risk-adapted maintenance approach in newly diagnosed multiple myeloma. J Clin Oncol. 2020;38:JCO.19.02515 https://doi.org/10.1200/JCO.19.02515
Richardson PG, Weller E, Lonial S, Jakubowiak AJ, Jagannath S, Raje NS, et al. Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly diagnosed multiple myeloma. Blood. 2010;116:679–86. https://doi.org/10.1182/blood-2010-02-268862
Parikh RHC, Almaula D, Heffner LT, Gupta VA, Boise L, Kaufman JL, et al. Updated survival with extended follow-up on patients with newly diagnosed multiple myeloma treated with lenalidomide, bortezomib, and dexamethasone (RVD) induction therapy and a risk-stratified maintenance approach. J Clin Oncol. 2022;40:1806
Voorhees PM, Sborov DW, Laubach J, Kaufman JL, Reeves B, Rodriguez C, et al. Addition of daratumumab to lenalidomide, bortezomib, and dexamethasone for transplantation-eligible patients with newly diagnosed multiple myeloma (GRIFFIN): final analysis of an open-label, randomised, phase 2 trial. Lancet Haematol. 2023;10:e825–e37. https://doi.org/10.1016/S2352-3026(23)00217-X
Sonneveld P, Dimopoulos MA, Boccadoro M, Quach H, Ho PJ, Beksac M, et al. Daratumumab, bortezomib, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2024;390:301–13. https://doi.org/10.1056/NEJMoa2312054
Palumbo A, Avet-Loiseau H, Oliva S, Lokhorst HM, Goldschmidt H, Rosinol L, et al. Revised international staging system for multiple myeloma: a report from International Myeloma Working Group. J Clin Oncol. 2015;33:2863–9. https://doi.org/10.1200/JCO.2015.61.2267
Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos MV, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15:e538–48. https://doi.org/10.1016/S1470-2045(14)70442-5
Moreau P, Hulin C, Zweegman S, Hashim M, Hu Y, Heeg B, et al. Comparative efficacy and safety of bortezomib, thalidomide, and dexamethasone (VTd) without and with daratumumab (D-VTd) in CASSIOPEIA versus VTd in PETHEMA/GEM in transplant-eligible patients with newly diagnosed multiple myeloma, using propensity score matching. EJHaem. 2021;2:66–80. https://doi.org/10.1002/jha2.129
Goldschmidt H, Mai EK, Bertsch U, Fenk R, Nievergall E, Tichy D, et al. Addition of isatuximab to lenalidomide, bortezomib, and dexamethasone as induction therapy for newly diagnosed, transplantation-eligible patients with multiple myeloma (GMMG-HD7): part 1 of an open-label, multicentre, randomised, active-controlled, phase 3 trial. Lancet Haematol. 2022;9:e810–e21. https://doi.org/10.1016/S2352-3026(22)00263-0
Costa LJ, Chhabra S, Medvedova E, Dholaria BR, Schmidt TM, Godby KN, et al. Minimal residual disease response-adapted therapy in newly diagnosed multiple myeloma (MASTER): final report of the multicentre, single-arm, phase 2 trial. Lancet Haematol. 2023;10:e890–e901. https://doi.org/10.1016/S2352-3026(23)00236-3
Leypoldt LB, Besemer B, Asemissen AM, Hanel M, Blau IW, Gorner M, et al. Isatuximab, carfilzomib, lenalidomide, and dexamethasone (Isa-KRd) in front-line treatment of high-risk multiple myeloma: interim analysis of the GMMG-CONCEPT trial. Leukemia. 2022;36:885–8. https://doi.org/10.1038/s41375-021-01431-x
Touzeau C, Perrot A, Hulin C, Manier S, Macro MD, Chretien ML, et al. Daratumumab Carfilzomib Lenalidomide and Dexamethasone with tandem transplant in high-risk newly diagnosed myeloma. Blood. 2024. https://doi.org/10.1182/blood.2023023597
Nooka AK, Kaufman JL, Muppidi S, Langston A, Heffner LT, Gleason C, et al. Consolidation and maintenance therapy with lenalidomide, bortezomib and dexamethasone (RVD) in high-risk myeloma patients. Leukemia. 2014;28:690–3. https://doi.org/10.1038/leu.2013.335
Author information
Authors and Affiliations
Contributions
Conception and design: NJ, JK, AN, SL; Provision of study materials/patients: NJ, JK, AN, VG, CH, SL; Collection and assembly of data: NJ, AN, SD, DR; Data analysis and interpretation: All authors; Manuscript writing: All authors; Final approval of manuscript: All authors; Accountable for all aspects of the work: All authors.
Corresponding author
Ethics declarations
Competing interests
Nisha S. Joseph has served on advisory boards and reports consultancy for Janssen Oncology, Bristol-Myers Squibb; research funding from Janssen, Bristol-Myers Squibb, Takeda, AstraZeneca, GSK. Jonathan L. Kaufman reports consultancy for and research funding from Janssen, Takeda, Bristol-Myers Squibb, Celgene, AbbVie, Roche, Seattle Genetics, Amgen, Sutro Biopharma, Karyopharm, Celgene, and Pharmacyclics outside the submitted work. Craig C. Hofmeister has served on an advisory board for Celgene and reports being the principal investigator of company-sponsored trials for Janssen, Celgene, Bristol-Myers Squibb, and Takeda-Millennium outside the submitted work. Ajay K. Nooka has served on advisory boards for Amgen, Spectrum Pharmaceuticals, Takeda Pharmaceuticals, Celgene, Bristol-Myers Squibb, GlaxoSmithKline, Adaptive Technologies, and Janssen Pharmaceuticals and reports grants from Amgen Pharmaceuticals, Takeda Pharmaceuticals, Bristol-Myers Squibb, Janssen Pharmaceuticals, Celgene, and GSK during the conduct of the study; he also reports grants from Novartis, Roche, and KITE outside the submitted work. Madhav V. Dhodapkar reports personal fees from Amgen and Janssen outside the submitted work. Sagar Lonial reports consultancy for and research funding from Takeda, Novartis, Bristol-Myers Squibb, GlaxoSmithKline, Amgen, Merck, Celgene, and Janssen outside the submitted work. The other authors made no disclosures.
Ethics approval and consent to participate
All data was de-identified and compiled as per our institutional IRB-approved protocol. Per IRB52278, we received a waiver of consent from Emory University IRB.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Joseph, N.S., Kaufman, J.L., Gupta, V.A. et al. Quadruplet therapy for newly diagnosed myeloma: comparative analysis of sequential cohorts with triplet therapy lenalidomide, bortezomib and dexamethasone (RVd) versus daratumamab with RVD (DRVd) in transplant-eligible patients. Blood Cancer J. 14, 159 (2024). https://doi.org/10.1038/s41408-024-01120-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41408-024-01120-9
- Springer Nature Limited