Abstract
The platypus is a semi-aquatic mammal, endemic to freshwater habitats of eastern Australia. There are gaps in the understanding of platypus movement behaviour within river systems, including spatial and temporal organization of individuals. We tracked movements of 12 platypuses on the regulated Snowy and Mitta Mitta Rivers for up to 12-months, the longest continuous tracking of platypus using acoustic telemetry. Platypuses remained relatively localized, occupying 0.73–8.45 km of river over 12 months, consistent with previous tracking studies over shorter periods. Males moved further than females, and larger males had higher cumulative movements, suggesting a possible relationship to metabolic requirements. Platypuses moved greater distances on the Mitta Mitta River, possibly associated with impacts of altered flow regimes to their macroinvertebrate diet. Increased movements and diurnal activity during winter were primarily driven by males, possibly attributable to breeding behaviours, rather than increased costs of winter foraging. Evidence for relatively small movements has implications for declining populations, given areas of localised declines are unlikely to be supplemented by migrating platypuses, especially when dispersal is restricted by dam walls. Understanding platypus movement behaviour is pertinent for their conservation, as water resource development and habitat modification continue to reduce connectivity between populations across their distribution.
Similar content being viewed by others
Introduction
Platypuses (Ornithorhynchus anatinus) occur mainly in creeks and rivers of eastern Australia, with an introduced population on Kangaroo Island1. They are primarily dependent on rivers and other water bodies, feeding exclusively on freshwater macroinvertebrates2, and using burrows on the water’s edge for resting and nesting3. Many aspects of platypus biology are increasingly well reported and studied, but movements, including spatial and temporal organisation of individuals, remains poorly understood4.
Linear home ranges of platypuses are typically < 15 km4, with sporadic long-distance movements5. Males generally have larger linear home ranges and move farther distances than females3,6,7, although lactating females may move greater distances8. There is overlap between male and female home ranges, reflecting their polygamous mating system3,5,9,10, with some males becoming territorial during breeding on some rivers9,10. Territorial behaviour generally occurs in late winter and is associated with increased testosterone and aggression11,12. Linear home ranges of juveniles are smaller than those of adults3, but male juveniles can move long distances during a dispersal phase13, as much as 44.4 km over a 30 week period14. Juveniles may be forced out of natal areas by adult resident platypuses15 or may disperse to reduce competition and avoid inbreeding14.
Platypuses are most active at night, but display diurnal activity on occasion9,10,16. They typically emerge from burrows within three hours of sunset7, with nightly foraging bouts spanning up to 11.3 km8. Foraging times also vary among individuals, particularly during breeding months9. Other evidence suggests that activity periods vary seasonally, with platypuses being more nocturnal during summer and autumn16 and diurnal activity increasing over the winter months6,9.
Long-distance, large-scale movements have been studied using mark-recapture surveys10, radiotelemetry3,7,10, data activity loggers16, and in-stream microchip readers17. However, mark-recapture surveys are labour intensive and recapture rates are low14,18,19, while other tracking methods (radiotelemetry, data activity loggers) are limited by battery life and attachment periods17, hindering continuous monitoring over long periods. Continuous long-term platypus tracking is essential for providing insights into changes in their movement behaviour. Acoustic transmitters can successfully track platypuses with externally attached transmitters, but there are attachment limitations20,21,22. Recently, intraperitoneal implants of acoustic transmitters have been used to provide data for up to six months (dependent on battery life6), suggesting this is an effective method for long-term continuous tracking of platypus movement.
In this study, we examined movement behaviour of platypuses on the regulated Snowy River (New South Wales) and the Mitta Mitta River (Victoria), using implanted acoustic transmitters and a fixed array of VR2W receivers. We investigated large-scale movements and present the longest continuous acoustic tracking time-series for individual platypuses yet reported. Data are used to evaluate differences in range, cumulative movements, and activity patterns among individuals in relation to demographic population structure and regulated river flows. We expected that males would have larger ranges and cumulative movements, particularly during the breeding season3,6,7. We also anticipated that juvenile males would move large distances during the study period, reflecting a potential dispersal phase13. Movements were expected to differ between rivers, reflecting differences in habitat, resources, and abundances.
Methods
Study sites
Platypus movements were investigated on the Snowy River, below Jindabyne Dam in south-eastern New South Wales and the Mitta Mitta River, below Dartmouth Dam in Victoria, Australia (Fig. 1). The Snowy River is characterised by deep pool and riffle sequences23, flowing through native eucalypt woodland (Eucalyptus spp.) in the Jindabyne Gorge and downstream adjoining grazing land24. It is regulated by the Jindabyne Dam, now receiving just 21% of its historic mean annual flow, increased from 1% after regulation25. Flows generally mimic the natural flow regime, with high flows over the spring (Stewardson & Gippel 2003). The Mitta Mitta River flows through a forested rocky gorge, emerging into cleared grazing land26. Downstream of Dartmouth Dam, the river is heavily regulated, with high summer flows diverted for irrigation27.
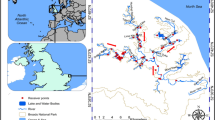
Locations of sites where acoustic transmitters were implanted in platypus, and locations of acoustic receivers used to detect their movements along the Snowy River (including two tributaries: M = Mowamba River and W = Wullwye Creek) and Mitta Mitta River (including one tributary: WC = Watchingorra Creek).
Trapping
We captured platypuses using fyke nets or unweighted mesh (gill) nets. Fyke nets (30 mm knotless 20 ply nylon, 1 m × 5 m wings and 0.8 m × 5 m wings) were used in streams with depths < 1 m and set in pairs, facing upstream and downstream to capture animals moving in both directions. Fyke net cod (distal) ends were tied to stakes, ensuring nets sat at least 30 cm above the water level, allowing platypuses to breathe. Fyke nets were set in the late afternoon and checked 3-hourly until sunrise. Mesh nets (80 mm multifilament nets, 25 m × 2 m) were used in pools usually with depths > 2 m and lengths ≥ 50 m, between dusk and 01:00 h. Nets were spotlighted every 2–3 min for platypus and physically examined hourly to remove possible snags. Platypuses were removed from nets and transferred to pillowcases for around 30 min prior to tagging. We captured platypuses at five sites on the Snowy River, and three sites on the Mitta Mitta River. On both rivers we attempted to have some platypus capture sites as close to the dam walls as possible, to ascertain the impact of regulated flows to platypus movement. Trapping and handling of platypuses was carried out in accordance with guidelines and approved by the NSW Office of Environment and Heritage (SL101655), NSW Department of Primary Industries (P15/0096-1.0 & OUT15/26392), and UNSW’s Animal Care and Ethics Committee (16/14A).
Movements
We used acoustic transmitters (Vemco Limited, Nova Scotia, Canada) and receivers (VR2W-069 k) to track platypus on the Snowy and Mitta Mitta Rivers. Acoustic transmitters broadcast a series of sound pulses, unique to each transmitter, which are detected by receivers placed in the water. On the Snowy River, implanted acoustic transmitters (V7-4L, 22.5 mm × 7 mm, weight in air 1.8 g, weight in water 1.0 g; 0.40% of average (± SD) juvenile female weight 0.46 ± 0.01 kg, 0.36% average juvenile male weight 0.50 ± 0.04 kg, 0.23% average female adult weight 0.80 ± 0.07 kg, 0.13% of average male adult weight 1.34 ± 0.16 kg; Table 1) were inserted in the peritoneal cavity of ten platypuses using anaesthesia, under established protocols reported in Bino et al. 2018. Platypuses were anaesthetised in the field using isoflurane (Pharmachem 5%) in oxygen (3 L/min) in an induction chamber, and then maintained using a T-piece facemask (1.5%, 1.0 L/min)28. To insert the transmitters, a small area of fur was removed from the ventral midline (5 mm × 15 mm longitudinally), halfway between the xiphisternum and the pubis. Prior to incision, 70% methanol was applied three times to the surgical site, followed by diluted chlorhexidine solution (0.1% weight/volume aqueous solution). A paper drape (5 mm × 15 mm) was attached and secured with glue at its edges. A 10 mm incision was made down to the linea alba, followed by an 8 mm incision into the peritoneum, both using a size-15 scalpel blade. The transmitter was flushed with sterile saline solution and then inserted into the peritoneal cavity, before the linea alba incision was closed with 3–4 single interrupted sutures. A few drops of bupivacaine hydrochloride were used as a local anaesthetic before the skin wound was closed with 1–2 cruciate sutures. Tissue adhesive (Vetbond) was also used to seal the incision. Platypuses were left to recover for a minimum of one hour before being returned to the water. Transmitters had a battery life expectancy of 197 days (~ 6.5 months).
To track implanted platypuses, 25 acoustic receivers were deployed along a 27 km stretch of river (February–August 2017, Fig. 1). Two receivers were placed above the Jindabyne Dam wall to ensure platypuses were detected if they were able to traverse the regulatory structure. Twelve receivers were concentrated for the first 5.5 km downstream of Jindabyne Dam wall (350–650-m intervals, average 475 ± 117 m), where the greatest numbers of platypus were trapped. The remaining receivers were located downstream at 3-km intervals (average 3 ± 0.23 km) to detect downstream long-distance movements and dispersal. Three receivers were placed on the Mowamba River tributary (350 m, 1.35 km and 2.35 km upstream from the junction with the Snowy River), and one receiver on the Wullwye Creek tributary (500 m from junction). Eight out of the ten platypuses with implanted transmitters were successfully detected and tracked over a 168-day period from February–August 2017 (Table 1). Two platypuses, (one juvenile male and juvenile female) failed to be detected after transmitters were implanted. Both platypuses were released into pools with a receiver, suggesting transmitter failure rather than an adverse outcome.
Despite considerable trapping effort on the Mitta Mitta River, only four platypuses were captured and tagged, with these captures not occurring directly downstream of the dam (minimum 8.4 km downstream; Fig. 1). These individuals were tagged with larger transmitters (V9-2L, 29 mm × 9 mm, weight in air 4.5 g, weight in water 2.7 g; 0.27% of average adult male weight 1.64 ± 0.26 kg; Table 1), which provided ~ 400 days (~ 13 months) of battery life. Twenty-five receivers were deployed to track the platypuses along a 32-km section of river below the Banimboola regulating pond (Fig. 1). A receiver was placed at each capture site, and two receivers placed at 500-m intervals upstream and downstream of the capture site (average 500 ± 100 m). One receiver was placed on Watchingorra Creek, 250 m upstream of the junction with the Mitta Mitta River. Remaining receivers were placed at 500-m–2-km intervals (average 1.4 ± 0.6 km), except for two receivers placed 5 km and 8.8 km further downstream to capture downstream large-scale movements or dispersal (Fig. 1). One receiver was lost during an extremely high flow event. All four platypuses were detected and tracked on the Mitta Mitta River.
Statistical analysis
We tested for variation in range and cumulative movements of platypuses among months (March–May) and between sexes. Daily range was calculated as the distance along the river between the most upstream and downstream detections by any receiver, measured along the middle of the river. Daily cumulative movements were calculated as the sum of distances between two consecutive detections between two receivers. We tested for differences in range and cumulative movements using generalised mixed-effects models (GLMMs), using a gamma error distribution with a log link29. As the gamma distribution requires positive values, we added 50 m to all measured distances, aligning with detections uncertainties of acoustic receivers for this species6,8,20,22. Prior to analysis, juvenile platypuses (Table 1) and all data from February and August on the Snowy River were removed, given limited number of detections (203 records from five individuals and 11 detections from four platypuses, respectively). We used the ‘glmer’ function in the ‘lme4’ package30 in the R environment31. We considered sex (factor with two levels) and month (factor with five levels), as well as the interaction between sex and month, as the predictors on the Snowy River, while we considered only month on the Mitta Mitta River, as only males were captured (Table 1). For both rivers, the identity of tagged platypuses was included as a random effect, given we aimed to make conclusions regarding the larger population, from which these individuals were a random sample6. We used post-hoc tests for differences between months based on the estimated marginal means, using the ‘emmeans’ package32 in the R environment31.
We also tested for any effects of flow rate (ML/d), rainfall amount (mm), and number of records on daily range and cumulative movements. Flow rates and rainfall for the Snowy River were collected from Dalgety Weir Gauge (222,026, 36.51° S, 148.83° E; Fig. 1) (BOM, NSW Water). Flow rates for the Mitta Mitta River were collected at Coleman’s Gauge (582,010, 36.53° S, 147.46° E), and rainfall data were from Callaghan Creek Station (36.45° S, 147.43° E). All flow rates were natural-log transformed to reduce skewness and improve normality. We excluded all data from February and August due to low numbers of detections. We used Generalized Additive Mixed Models (GAMMs), offering a compromise between a linear model and a smoothing function, making them more flexible with fewer assumptions33. In GAMM models, splines are a collection of simple polynomial functions that are joined in locations known as knots. The flexibility of the spline curve is therefore determined by the number of knots specified in the model. Large numbers of knots can cause over-fitting of the model and so to avoid this, we limited the number of knots in the GAMMs to three34. We used the ‘gamm’ function in the ‘gamm4’ package35, in the R environment31. Similarly to the GLMMs, we used a gamma log link distribution and added 50 m to estimated distances. Daily range or cumulative movements were included as the response variable, and number of records, month (continuous), sex (two levels), flow rate (ML/d) and rainfall (mm) were included as predictors. We also included individual platypuses as a random effect, and an interaction term between sex and month to explore variation in monthly movements between sexes.
To investigate variation in hourly activity patterns, we calculated the percentage of nocturnal records, defined as those that occurred between one hour before sunset and one hour after sunrise. Average sunset/sunrise times were calculated from Jindabyne for the Snowy River and Albury for the Mitta Mitta River.
Results
General observations
Number of tracking days for the eight detected individuals on the Snowy River ranged from 144–158 (average 153 days). Number of detections ranged from 24 to 5554 (adult female average (± sd) 1950 ± 690, adult male average 2512 ± 2731, juvenile female 531, juvenile male 24) (Table 1). Platypuses were detected at 13/25 receivers and were not detected by receivers beyond 8.9 km downstream of the dam wall (Fig. 1). On the Mitta Mitta River, platypuses were detected for 134–345 days (average 255 days). Number of detections ranged from 3180 to 6079 (average 4134 ± 1318), with platypus being detected at 16/25 receivers (Fig. 1).
Range movements
Most platypuses were detected both upstream and downstream of their initial tagging location (Table 1), with river position varying for each individual over time (Appendix 1). On the Snowy River, eight platypuses with implanted transmitters utilised between 0.73 and 4.76 km of river (Fig. 2a; Table 1; Appendix 1). The average daily range for all individuals was 0.39 ± 0.66 km. MA2 had the highest average (1.10 ± 1.04 km) and maximum (4.35 km) daily river range and was detected over a total linear river range of 4.76 km. The male juvenile (MJ1) had the smallest movement range of tracked platypuses, with a range of 730 m over 151 days, but was detected far less often than other platypuses in the study (Table 1). The average daily range for male adults was greater than for female adults, significantly higher in all months (P ≤ 0.020) except March (z = − 1.554, P = 0.120) and April (z = − 1.461, P = 0.144; Fig. 2b, Appendix 2). For females, average daily ranges were significantly higher in April (0.26 ± 0.19 km) compared to all other months (P ≤ 0.041) and significantly lower in June (0.07 ± 0.11 km) compared to all other months (P ≤ 0.001) except July (0.12 ± 0.25 km, z = − 1.689, P = 0.072), (Fig. 2b; Appendix 2). For males, movement ranges peaked in the winter month of July (0.94 ± 1.22 km), significantly higher than March and June (P ≤ 0.049) and marginally higher compared to April and May (P ≤ 0.093), (Fig. 2b; Appendix 2). Based on GAMM models, daily range of platypus movements had a significant positive association with number of detections up to 100 detections but declined thereafter (F = 16.911, P < 0.001), a significant decreasing effect of flow rates (ML/d) (F = 4.343, P = 0.037; Appendix 2) and no associations with rainfall (mm) (F = 0.506, P = 0.477).
On the Mitta Mitta River, the daily average range of the four males was 1.0 ± 0.46 km, with platypuses utilising 1.84–8.45 km of river over the 12-month period. MA12 and MA13 had far greater movements upstream and downstream, compared to MA14 and MA15 (Fig. 2a; Table 1). MA12 had the highest average daily range (1.39 ± 1.00 km), while MA13 had the highest maximum daily range (3.50 km) and used a linear river range of 8.45 km during the 12-month period. Range movements were significantly higher in June (1.26 ± 0.75 km), July (1.46 ± 0.91 km), and August (1.37 ± 0.75 km), compared with all other months (P ≤ 0.001; Fig. 2b, Appendix 2). Based on GAMM models, daily range movements had a significant positive association with number of detections (F = 48.919, P < 0.001, Appendix 2), and flow (F = 11.899, P < 0.001, Appendix 2), increasing towards 1900 ML/d, but decreasing with greater flow rates. No association were detected with rainfall (F = 0.946, P = 0.331, Appendix 2).
Cumulative movements
On the Snowy River, average cumulative daily movements were 0.87 ± 1.18 km. MA2 had the highest average (2.37 ± 1.57 km) and maximum (9.04 km) daily cumulative movements and travelled 335.30 km over 155 days (Fig. 3a). Adult males had significantly higher cumulative daily movements compared to females for all months (P ≤ 0.004) except April (z = − 0.582, P = 0.560; Fig. 3b, Appendix 3). Cumulative daily movements of females were higher in April (0.65 ± 0.49 km) compared to all months (P ≤ 0.017) and significantly lower in June (0.19 ± 0.29 km) compared to all months (P ≤ 0.001) except July (0.27 ± 0.50 km, z = 1.400, P = 0.162). Male cumulative movements were significantly higher in July (2.03 ± 1.99 km) compared to April (1.30 ± 1.14 km, z = − 2.575, P = 0.010), and marginally higher than in March, May, and June (P ≤ 0.085; Appendix 3). Based GAMM models, the number of detections was positively associated with range up to 100 detections but declined thereafter (F = 21.398, P < 0.001), a negative association with flow (F = 4.254, P = 0.029), and no association with rainfall (F = 0.052, P = 0.820), (Appendix 3).
On the Mitta Mitta River, average cumulative daily movements were 2.43 ± 1.22 km. MA12 had the highest average (3.48 ± 2.46 km) and maximum (9.61 km) daily cumulative movements and travelled 779.14 km over the 12-month period (Fig. 3a). Cumulative movements were significantly higher in June (2.75 ± 1.75 km), July (3.44 ± 2.23 km), and August (3.73 ± 2.12 km), compared with all other months (P ≤ 0.001; Fig. 3b, Appendix 3). Based on GAMM models, cumulative daily movements were significantly associated with number of detections (F = 61.704, P < 0.001). Cumulative daily movements had a positive association with flow, increasing towards 1900 ML/d, before a slight decrease (F = 24.124, P < 0.001) and a significant positive association with rainfall (F = 5.235, P = 0.022; Appendix 3).
Activity patterns
On the Snowy River, platypuses varied their daily activity patterns each month (Fig. 4, Table 2). Platypuses displayed mostly primarily nocturnal activity. This was most pronounced in April, with 93.2% of detections between one hour before sunset and one hour after sunrise (henceforth ‘night’) (average time of sunset 17:37, average time of sunrise 06:31). Nocturnal activity was less common during June, with 67.8% of detections at night (15:56, 08:15). There was variation between sexes, with females exhibiting more nocturnal behaviour than males during March, April, and July (Table 2).
On the Mitta Mitta River, platypuses were most nocturnal in June (98.6% of detections at night; 17:07, 7:20) and April (95.3% of detections between 17:58, 6:49), but nocturnal behaviour was prominent for most months (Table 2). Platypuses were least nocturnal during the spring months, particularly in September (60.4% of detections at night; 17:01 and 7:12).
Discussion
Tracking movements of mobile and aquatic animals is critical for understanding their life history and behaviour, resulting in better management of their threats36,37,38, and how they respond to changes in environmental variables39. We tracked movements of twelve platypuses on the Snowy and Mitta Mitta Rivers, using implanted acoustic transmitters for up to 12-months. There were consistencies between movement behaviour in this study and previously reported movements for platypuses, but range, cumulative movements, and activity also varied between sexes, among months, and potentially in response to river regulation. There was also some variation between rivers, likely reflecting differences in social organisation, competition, habitat, and the availability of local resources5,7,9,10,40.
Similar to previous studies, platypuses were relatively localised, with their movements, remaining within a few kilometres of their initial tagging location, despite the longer period over which we monitored individuals. Previously, studies suggested the maximum linear range male platypuses effectively patrol to be around 7 km, resulting in a maximum movement of 14 km in a 24-h period for a return trip5. Although our sample size was small, this was similar to our maximum linear range of 8.45 km, and distances of 0.5–15 km for tracked platypuses on other rivers4. Movement, site fidelity, and home range in mammals is influenced by accessibility of mates41 and resources42. Site fidelity may also be beneficial in populations with male–male conflict, with resident males shown to have an advantage over intruders43,44. Platypuses likely confine themselves to these ranges because mates and adequate food sources are available.
While there were some consistencies in movement, there was also variation, particularly between sexes and age groups. Females moved less than males on the Snowy River and were not detected more than 1.5 km from their initial tagging location, consistent with platypus behaviour on other rivers3,7. Longer movements in males is likely due to territorial and mate acquisition requirements during the breeding season14,16, but we also identified these patterns over the non-breeding months (Fig. 4b). Interestingly, the largest males moved furthest on the Snowy River and Mitta Mitta River (Figs. 2a, 3a), indicating some possible positive relationship between increasing body size and movements, not apparent from the Goulburn River, Victoria9, although this could not be tested in this study due to the low sample size of individuals. The relationship between increasing movements and body mass occurs in different mammals45, birds and lizards46, and turtles47. It may be related to higher energetic requirements in larger animals48 and the need to forage more widely14.
Despite high sampling effort, no juveniles were tagged on the Mitta Mitta River, and two of the four tagged juveniles on the Snowy River could not be tracked, limiting our capacity to assess juvenile dispersal. In mammals, dispersal tends to be male-based49, with juvenile males of many species dispersing away from the natal area after weaning50,51. Juvenile male platypuses can travel long distances from natal sites6,13,14, which probably reduces competition and inbreeding, and facilitates access to new home ranges. We tracked two juveniles on the Snowy River, with the male juvenile (MJ1) travelling the smallest distances (Figs. 2a, 3a). MJ1 was detected at only a single receiver (660.13 m downstream of the Jindabyne Dam wall, Fig. 1), suggesting limited movements, before he moved 400 m downstream of his resident pool on a single day, during the final week of the study in July. Potentially, this might represent the initiation of his dispersal phase, but this cannot be robustly inferred from this study. Movements upstream were also probably not possible as he was captured within a kilometre of the Jindabyne Dam wall, likely impacting dispersal for this platypus52,53.
Platypuses also varied their movements with time of year, with differences between sexes and subtle differences between rivers. Platypuses generally move further during the winter months16, perhaps because of increased thermoregulatory costs (16–20% higher during winter) and reduced diversity and size of benthic organisms54. However, in this study males increased their movements over the winter months on the Snowy and Mitta Mitta Rivers, but both range and cumulative movements were lower in winter than in the autumn months for females (Figs. 2b, 3b). If platypuses were increasing their range and cumulative movements to counteract the costs of winter foraging, we would expect this to be consistent across sexes. Given increases were more likely for males, this may reflect increased efforts to establish territories and locate females prior to breeding9. Platypuses have also been shown to increase diurnal activity over the winter months6,9. In Tasmania, increased diurnal activity occurred primarily in females16, but males were more active in the day than females during July on the Snowy River, complementing increased movements in the lead up to the breeding season4.
While there were some similarities in movement and activity patterns on both rivers, there were also some notable differences. Average daily cumulative movements on the Mitta Mitta River were higher than on the Snowy River. These differences may reflect possible scarcity of habitat and resources downstream of Dartmouth Dam on the Mitta Mitta River. Due to extensive regulation, water temperatures downstream of Dartmouth Dam can be as much as 12 °C below normal55, which may reduce macroinvertebrate food availability downstream of the dam26,56, also reflected in reduced abundances of platypuses compared to capture rates on the Snowy River57. There was some evidence that flows were associated with both range and cumulative movements, suggesting that platypuses may reduce foraging distances under higher flows due to increased energetic demands8. Identifying how flows interact with prey abundance and platypus movements, remains a critical gap in understanding habitat requirements and estimating population sizes.
This study was limited by the small sample sizes (number of tracked platypuses) and limited replication (number of rivers), limiting the scope of inference about long-term movements of platypus, particularly in relation to sex and life stage. Although significant effort was made, the number of tracked platypuses was particularly small on the Mitta Mitta River, with only four males captured. The small numbers of tagged platypuses also limited comparisons between the two river systems, but this probably reflected the poor state of the platypus population on the Mitta Mitta River57. Movement tracking of platypuses on both rivers occurred for periods of 6–12 months, some of the longest movement tracking data for this species. However, this was still not long enough to adequately represent annual variation of movement behaviours in relation to breeding and resource availability. Some variation probably also occurred among rivers due to the fixed placement of acoustic receivers, potentially resulting in underestimation of ranges and cumulative movements of platypuses foraging in areas with no receivers. These limitations are inevitable when studying a cryptic freshwater species found in low densities.
Platypus movements are increasingly understood, particularly the existence of restricted ranges, after potential dispersal phases for male juveniles. This has implications for declining populations58,59, given that populations in areas of local declines and extinctions are unlikely to be supplemented by migrating platypuses. This is particularly problematic if dispersing juveniles are restricted by dam walls, potentially impacting metapopulation connectivity and the future viability of populations60. While there was no strong evidence of impacts of changing regulated flows to movements, platypus movements may be indirectly influenced by the impacts of river regulation on abundance of their macroinvertebrate prey. Future research would benefit from tracking platypuses to assess habitat use and quality, and the relationship this has with their macroinvertebrate food sources. Understanding platypus movements, particularly on regulated systems, is increasingly important for their conservation, given ongoing declines and drying of water bodies across their distribution.
References
Grant, T. & Denny, M. J. S. Distribution of the platypus in Australia with guidelines for management. A report to Australian National Parks and Wildlife Service. Mount King Ecological Surveys, Oberon, New South Wales (1991).
Serena, M., Worley, M., Swinnerton, M. & Williams, G. Effect of food availability and habitat on the distribution of platypus (Ornithorhynchus anatinus) foraging activity. Aust. J. Zool. 49, 263–277 (2001).
Serena, M., Thomas, J., Williams, G. & Officer, R. Use of stream and river habitats by the platypus, Ornithorhynchus anatinus, in an urban fringe environment. Aust. J. Zool. 46, 267–282 (1998).
Bino, G. et al. The platypus: evolutionary history, biology, and an uncertain future. J. Mammal. 100, 308–327 (2019).
Gardner, J. & Serena, M. Spatial-organization and movement patterns of adult male platypus, Ornithorhynchus anatinus (Monotremata, Ornithorhynchidae). Aust. J. Zool. 43, 91–103 (1995).
Bino, G., Kingsford, R. T., Grant, T., Taylor, M. D. & Vogelnest, L. Use of implanted acoustic tags to assess platypus movement behaviour across spatial and temporal scales. Sci. Rep. 8, 5117 (2018).
Grant, T., Grigg, G. C., Beard, L. & Augee, M. Movements and burrow use by platypuses, Ornithorhynchus anatinus, in the Thredbo River, New South Wales. In Platypus and Echidnas (ed. Augee, M. L.) 263–267 (The Royal Zoological Society of NSW, Sydney, 1992).
Griffiths, J., Kelly, T. & Weeks, A. Impacts of high flows on platypus movements and habitat use in an urban stream. Report to Melbourne Water. Cesar, VIC, Australia (2014).
Gust, N. & Handasyde, K. Seasonal-variation in the ranging behavior of the platypus (Ornithorhynchus anatinus) on the Goulburn River, Victoria. Aust. J. Zool. 43, 193–208 (1995).
Serena, M. Use of time and space by platypus (Ornithorhynchus anatinus: Monotremata) along a Victorian stream. J. Zool. 232, 117–131 (1994).
Temple-Smith, P. & Grant, T. Uncertain breeding: a short history of reproduction in monotremes. Reprod. Fertil. Dev. 13, 487–497 (2001).
Temple-Smith, P. D. Seasonal breeding biology of the platypus, Ornithorhynchus anatinus (Shaw, 1799), with special reference to the male (1973).
Bino, G., Grant, T. R. & Kingsford, R. T. Life history and dynamics of a platypus (Ornithorhynchus anatinus) population: four decades of mark-recapture surveys. Sci. Rep. 5, 16073 (2015).
Serena, M. & Williams, G. A. Movements and cumulative range size of the platypus (Ornithorhynchus anatinus) inferred from mark-recapture studies. Aust. J. Zool. 60, 352–359 (2012).
Grant, T. Body temperatures of free-ranging platypuses, Ornithorhynchus anatinus (Monotremata), with observations on their use of burrows. Aust. J. Zool. 31, 117–122 (1983).
Bethge, P., Munks, S., Otley, H. & Nicol, S. Activity patterns and sharing of time and space of platypuses, Ornithorhynchus anatinus, in a subalpine Tasmanian lake. J. Mammal. 90, 1350–1356 (2009).
Macgregor, J. et al. Novel use of in-stream microchip readers to monitor wild platypuses. Pac. Conserv. Biol. 21, 101–101 (2015).
Grant, T. Captures, capture mortality, age and sex ratios of platypuses, Ornithorhynchus anatinus, during studies over 30 years in the upper Shoalhaven River in New South Wales. Proc. Linn. Soc. NSW 125, 217–226 (2004).
Grant, T. & Temple-Smith, P. Conservation of the platypus, Ornithorhynchus anatinus: threats and challenges. Aquat. Ecosyst. Health Manag. 6, 5–18 (2003).
Griffiths, J., Kelly, T. & Weeks, A. Net-avoidance behaviour in platypuses. Aust. Mammal. 35, 245–247 (2013).
Griffiths, J. & Weeks, A. Impact of environmental flows on platypuses in a regulated river. Report to Melbourne Water. Cesar, VIC, Australia (2015).
Hawke, T. et al. Fine-scale movements and interactions of platypuses, and the impact of an environmental flushing flow. Freshw. Biol. 2020(00), 1–12 (2020).
Erskine, W. et al. Bedform maintenance and pool destratification by the new environmental flows on the Snowy River downstream of the Jindabyne Dam, New South Wales. J. Proc. R. Soc. N. S. W. 150, 152–171 (2017).
Rohlfs, A. M. Rehabilitating the Snowy River: The influence of environmental flow releases on dissolved organic carbon supply and utilisation. PhD thesis, University of Technology, Sydney, Australia (2016).
Pigram, J. J. Options for rehabilitation of Australia’s Snowy River: an economic perspective. Regul. Rivers Res. Manag. 16, 363–373 (2000).
Davey, C. Mitta Mitta Biological Monitoring Program 2013–2014 Annual Report. Annual report prepared for the Murray Darling Basin Authority by The Murray–Darling Freshwater Research Centre, MDFRC Publication 45/2014, September, 88pp. Canberra, Australia (2014).
Watts, R. J., Ryder, D. S. & Allan, C. Environmental monitoring of variable flow trials conducted at Dartmouth Dam, 2001/02-07/08-Synthesis of key findings and operational guidelines. Institute for Land, Water and Society Report Number 50, Charles Sturt University, Albury, NSW (2009).
Vogelnest, L. & Woods, R. Medicine of Australian Mammals (CSIRO Publishing, Clayton, 2008).
McCullagh, P. & Nelder, J. A. Generalized Linear Models 2nd edn. (Chapman and Hall, London, 1989).
Bates, D. et al. Package “lme4”. Convergence 12, 2 (2015).
R Development Core Team. R: a language and environment for statistical computing (R Foundation for Statistical Computing, 2020).
Lenth, R. emmeans: Estimated Marginal Means, aka Least-Squares Means. R package version 1.3.0. https://CRAN.R-project.org/package=emmean.
Wood, S. N. & Augustin, N. H. GAMs with integrated model selection using penalized regression splines and applications to environmental modelling “Manuscript”. Ecol. Model. 157, 157–177 (2002).
Wood, S. N. Generalized Additive Models: An Introduction with R (Chapman and Hall/CRC, Boca Raton, 2017).
Wood, S., Scheipl, F. & Wood, M. S. Package ‘gamm4’. Am. Stat. 45, 339 (2017).
Hazen, E. L. et al. Ontogeny in marine tagging and tracking science: technologies and data gaps. Mar. Ecol. Prog. Ser. 457, 221–240 (2012).
McClellan, C. M. & Read, A. J. Complexity and variation in loggerhead sea turtle life history. Biol. Lett. 3, 592–594 (2007).
Taylor, M. D., Babcock, R. C., Simpfendorfer, C. A. & Crook, D. A. Where technology meets ecology: acoustic telemetry in contemporary Australian aquatic research and management. Mar. Freshw. Res. 68, 1397–1402 (2017).
Pillans, R., Fry, G., Steven, A. & Patterson, T. Environmental influences on long-term movement patterns of a euryhaline Elasmobranch (Carcharhinus leucas) within a subtropical estuary. Estuar. Coasts 43, 2152–2169 (2020).
Otley, H. M., Munks, S. A. & Hindell, M. A. Activity patterns, movements and burrows of platypuses (Ornithorhynchus anatinus) in a sub-alpine Tasmanian lake. Aust. J. Zool. 48, 701–713 (2000).
Hendry, A. P. et al. Evolution Illuminated: Salmon and Their Relatives (Oxford University Press, Oxford, 2003).
Stevick, P. et al. Population spatial structuring on the feeding grounds in North Atlantic humpback whales (Megaptera novaeangliae). J. Zool. 270, 244–255 (2006).
Cutts, C., Brembs, B., Metcalfe, N. & Taylor, A. Prior residence, territory quality and life-history strategies in juvenile Atlantic salmon (Salmo salar L.). J. Fish Biol. 55, 784–794 (1999).
Haley, M. P. Resource-holding power asymmetries, the prior residence effect, and reproductive payoffs in male northern elephant seal fights. Behav. Ecol. Sociobiol. 34, 427–434 (1994).
Lindstedt, S. L., Miller, B. J. & Buskirk, S. W. Home range, time, and body size in mammals. Ecology 67, 413–418 (1986).
Turner, F. B., Jennrich, R. I. & Weintraub, J. D. Home ranges and body size of lizards. Ecology 50, 1076–1081 (1969).
Slavenko, A., Itescu, Y., Ihlow, F. & Meiri, S. Home is where the shell is: predicting turtle home range sizes. J. Anim. Ecol. 85, 106–114 (2016).
McNab, B. K. Bioenergetics and the determination of home range size. Am. Nat. 97, 133–140 (1963).
Greenwood, P. J. Mating systems, philopatry and dispersal in birds and mammals. Anim. Behav. 28, 1140–1162 (1980).
Soderquist, T. R. & Serena, M. Juvenile behaviour and dispersal of chuditch (Dasyurus geoffroii) (Marsupialia: Dasyuridae). Aust. J. Zool. 48, 551–560 (2000).
Cockburn, A., Scott, M. P. & Scotts, D. J. Inbreeding avoidance and male-biased natal dispersal in Antechinus spp. (Marsupialia: Dasyuridae). Anim. Behav. 33, 908–915 (1985).
Furlan, E. et al. Dispersal patterns and population structuring among platypuses, Ornithorhynchus anatinus, throughout south-eastern Australia. Conserv. Genet. 14, 837–853 (2013).
Kolomyjec, S. H. Relationships of northern platypus (Ornithorhynchus anatinus) populations: a molecular approach (2010).
Faragher, R., Grant, T. & Carrick, F. Food of the platypus (Ornithorhynchus anatinus) with notes on the food of brown trout (Salmo trutta) in the Shoalhaven River, NSW. Aust. J. Ecol. 4, 171–179 (1979).
Todd, C. R., Ryan, T., Nicol, S. J. & Bearlin, A. R. The impact of cold water releases on the critical period of post-spawning survival and its implications for Murray cod (Maccullochella peelii peelii): a case study of the Mitta Mitta River, southeastern Australia. River Res. Appl. 21, 1035–1052 (2005).
Koehn, J., Doeg, T., Harrington, D. & Milledge, G. The effects of Dartmouth Dam on the aquatic fauna of the Mitta Mitta River. Report to the Murray-Darling Basin Commission. Arthur Rylah Institute for Environmental Research, Melbourne (1995).
Hawke, T., Bino, G., & Kingsford, R. T. Damming insights: impacts and implications of river regulation on platypus populations. Aquat Conserv Mar Freshw Ecosystems. in press. (2021).
Hawke, T., Bino, G. & Kingsford, R. T. Platypus: paucities and peril. A response to: limitations on the use of historical and database sources to identify changes in distribution and abundance of the platypus. Glob. Ecol. Conserv. 21, e00857 (2019).
Hawke, T., Bino, G. & Kingsford, R. T. A silent demise: historical insights into population changes of the iconic platypus (Ornithorhynchus anatinus). Glob. Ecol. Conserv. 20, e00720 (2019).
Bino, G., Kingsford, R. T. & Wintle, B. A. A stitch in time—synergistic impacts to platypus metapopulation extinction risk. Biol. Conserv. 242, 108399 (2020).
Acknowledgements
This study was funded by ARC Linkage LP150100093, the Taronga Conservation Society, and the Australian Government’s Environmental Water Holder. Trapping and handling of platypuses was carried out in accordance with guidelines and approved by the NSW Office of Environment and Heritage (SL101655), NSW Department of Primary Industries (P15/0096-1.0 & OUT15/26392), and UNSW’s Animal Care and Ethics Committee (16/14A).
Author information
Authors and Affiliations
Contributions
T.H., G.B., and R.T.K. designed the study, M.D.T., K.I., and D.I. provided equipment and support for the study, T.H. and G.B. collected and analysed the data, T.H. wrote manuscript with contributions from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hawke, T., Bino, G., Kingsford, R.T. et al. Long-term movements and activity patterns of platypus on regulated rivers. Sci Rep 11, 3590 (2021). https://doi.org/10.1038/s41598-021-81142-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-81142-6
- Springer Nature Limited
This article is cited by
-
Effect of dam construction on changes in river's environmental flow (case study: Gorganrood river in the south of the Caspian Sea)
Applied Water Science (2023)
-
Using DNA metabarcoding as a novel approach for analysis of platypus diet
Scientific Reports (2022)
-
Fragmentation by major dams and implications for the future viability of platypus populations
Communications Biology (2022)
-
Seasonal and geographic variation in packed cell volume and selected serum chemistry of platypuses
Scientific Reports (2021)