Abstract
Implant outcomes in comparison to a natural tooth in a rat model using systemic alendronate and zoledronate acid drug administrations were assessed. Fifty-four Sprague–Dawley rats were randomly allocated into two experimental groups (drug application of zoledronic acid; 0.04 mg/kg intravenously once a week and alendronic acid; 0.2 mg/kg subcutaneously five times a week) and one control group with 18 animals in each group. Drug delivery was conducted for a period of 4 months. After 4 weeks either a zirconia or a titanium implant was immediately inserted in the socket of the first molar of the upper jaw. In vivo investigations included host inflammatory parameters and the implant survival and success rates for up to 3 months. Material incompatibilities against titanium and zirconia nanoparticles were evaluated in vitro after stimulation of rat spleen cells. In vivo, IL-6 release around titanium implants demonstrated significantly higher values in the control group (p = 0.02) when compared to the zoledronic acid group. Around the natural tooth without drug administration, the control group showed higher IL-6 values compared with the alendronic acid group (p = 0.01). In vitro, only lipopolysaccharide and not the implant’s nanoparticles stimulated significant IL-6 and TNFα production. In terms of the primary aim of in vivo and in vitro IL-6 and TNFα measurements, no implant material was superior to the other. No significant in vitro stimulation of rat spleen cells was detected with respect to titanium oxide and zirconium oxide nanoparticles.
Similar content being viewed by others
Introduction
In clinical dental practice, dentists increasingly encounter patients who regularly take bisphosphonates1. Osteoblasts and osteoclasts communicate with each other via different signaling molecules2. The osteoblasts produce a protein called RANKL, which after binding to the RANK receptor on the osteoclast activates the osteoclasts so that bone is resorbed2. Bisphosphonates are pyrophosphate analogues in which oxygen is substituted by carbon in the P-O-P bond3. As a result, no enzymatic hydrolysis occurs in the body. Bisphosphonates have a very high affinity for bone mineral because they bind to hydroxyapatite crystals4. Medication-related osteonecrosis of the jaw (MRONJ) has been described as a side effect5. Different pathologies have been discussed in the development of MRONJ6. On the one hand, reduced bone remodeling was described as the main cause. The inhibition of farnesyl-diphosphatase inhibits osteoclasts and leads to an increased rate of apoptosis of the osteoclasts2. Bisphosphonates also promote the reduction of vascular cells and thus the development of avascular necrosis, since the vascular supply is essential for a vital bone metabolism7,8. The medicinal effect influences not only the bone but also the overlying soft tissue, which explains the accompanying wound-healing disorders in these patients7,9. Systemic dosages are also perceived to have effects on the osteoblasts, which are also inhibited7. Not only bisphosphonates, but also other drugs, such as monoclonal antibodies, vascular endothelial growth factors, and tyrosine kinase inhibitors, have been related to MRONJ6,10,11. The prevalence of MRONJ, depending on the underlying disease and the type of drug administration, could be as high as 20% in intravenous injections in the case of malignant underlying disease. By contrast, oral drug administrations lead to low risk profiles; for example, primary osteoporosis at approximately 0.1%7. Bisphosphonates have a very long pharmacological half-life in the bone: in some cases more than ten years, which means that the indication for the use of this active substance should be strict5. Implant placement can be a risk factor. There are numerous case series of osteonecrosis associated with bisphosphonate after implantation or retrospective studies of bisphosphonate-associated osteonecrosis that have observed a relationship between osteonecrosis and bisphosphonate12,13,14,15,16. On the other hand, a number of studies have not observed any such connection17,18,19,20,21. The alternative therapy to implantological solutions often represents a purely tegumentally supported dental prosthesis, but here too, studies show a clear relationship between prostheses and their pressure points to the occurrence of osteonecrosis22,23,24. These studies only included titanium implants. Whether modern zirconium dioxide implants have advantages due to other material properties, such as a reduced tendency to mucositis, remains unclear25,26. It is of great importance to learn more about modern zirconia implants, especially with regard to the material properties and the resulting individual peri-implant inflammation risk. Proinflammatory cytokines such as TNF alpha, that promotes bone resorption and mediates the inflammatory response to infection, can be used to evaluate the level of inflammation27. The connection between soft tissue inflammation and the expression of inflammation-associated biomarkers around the teeth and implants has been demonstrated previously28,29. The purpose of this rat study was to investigate the peri-implant interface around titanium and zirconia implants in a high-risk group with systematic antiresorptive bisphosphonate drug administration. The primary aim was to assess immunological parameters, such as interleukin-6 (IL-6) and tumor necrosis factor alpha (TNFα), after immediate implant insertion in comparison to the natural tooth. The secondary aim was to conduct an in vitro stimulation test to evaluate hypersensitivity reactions to titanium and zirconium oxide nanoparticles. Furthermore, implant success rates and clinical parameters were evaluated.
Material and methods
Experimental protocol
The local authority office has approved the study. Fifty-four adult male Sprague–Dawley rats with a weight of 250 g and aged 7 weeks (Janvier Labs, Le Genest-Saint-Isle, France) were included in this study. This investigation was conducted in accordance with the guidelines of the European Parliament and of the Council on the protection of animals used for scientific purposes, Directive 2010/63 EU and was related to the immunological and clinical findings. We confirm that all experiments were performed in accordance with relevant named guidelines and regulations and the study complied with the ARRIVE guidelines. The study protocol was approved by the appropriate local authority (Landesamt für Natur und Verbraucherschutz, Recklinghausen, Germany; Ref. 2018A314). According to the laws in Germany § 15 TierSchG, the local ethic committee of the RWTH university does not provide permission to animal studies in Nord-Rhein Westfalen, Germany, only the LANUV https://www.lanuv.nrw.de/verbraucherschutz/tierversuche.
A single examiner performed each assessment for clinical and immunological diagnosis during the entire period of the study. Two experimental groups and one control group with 18 animals in each group were randomly divided as follows: one group treated with zoledronic acid, one group treated with alendronic acid, and one control group without any drug administration. Systemic drug administration with antiresorptive drugs was conducted for a period of 4 months and was started 4 weeks before surgery (Fig. 1). The drugs were diluted with physiologic phosphate-buffered saline before application. The zoledronic acid group received a dose of 0.04 mg/kg of their body weight of zoledronic acid (Mylan dura GmbH, Darmstadt, Germany) intravenously in the tail vein once every week30. In the second test group, a total of 0.2 mg/kg body weight of alendronic acid (Alendronate sodium trihydrate, Sigma Aldrich GmbH, Munich, Germany) was applied subcutaneously five times a week31. The rats were provided with food and water ad libitum, with only soft soaked food administered after implantation until the end of the investigation.
Implant placement
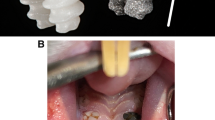
A total of 54 microrough titanium and 54 zirconia implants with a polished shoulder (length of 4 mm and diameter of 2 mm) were custom-made by Straumann Company with the same process used on commercially available implants (Institute Straumann AG, Basel, Switzerland).
After 4 weeks of drug delivery, the rats received an intraperitoneal anesthetic cocktail consisting of 90 mg/kg of body weight of ketamine (Medistar GmbH, Ascheberg, Germany) and 0.2 mg/kg of body weight of medetomidine hydrochloride (Domitor, Bayer Austria, Wien, Austria). Subsequently, after extraction of the first molar of the upper jaw on each side, either a zirconia or a titanium implant was immediately inserted using a randomization of the side distribution (Fig. 2). The insertion process included a pilot drill with a 2.2-mm diameter (Institute Straumann AG, Basel, Switzerland) and was performed according to the manufacturer’s protocol using a screwdriver, a torque of 15–20 Ncm, and a transgingival healing process. At the end of the surgery, the antidote atipamezole hydrochloride (Orion Pharma, Espoo, Finland), at a dose of 0.8 mg/kg body weight, was applied subcutaneously. In the first postoperative 3 days, the animals were visited and treated once a day with carprofen (4 mg/kg) subcutaneously (Rimadyl, Zoetis GmbH, Berlin, Germany) according to a score sheet.
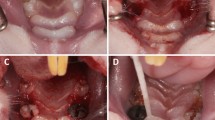
(A) Intraoral view of the upper jaw. The clinical probing pocket depth was measured with a dental probe. (B) In vivo material were collected with sterile color-coded paper points. (C) The sulcus bleeding index was evaluated around each implant (SBI around the titanium implant was 3 = severe bleeding). (D) In this case due to peri-implantitis and loss of stability, the titanium implant had to be removed.
In vivo immunological and clinical examination
In vivo sample collection and clinical inspection of the rats were conducted under inhalation anesthesia with isofluran (2.5–5 vol.% Piramal GmbH, Hallbergmoos, Germany) at three different time points (1 week, 8 weeks, and 12 weeks after surgery, Fig. 1).
Before the sample collection, under sterile conditions, the paper points with color-coded ends were cut off at the lowest colored end, according to the procedure of a previously published study25. Afterwards, sterile paper points were inserted at the deepest probing pocket depth around each implant (either zirconia or titanium) or natural tooth (second molar of one side), which was the deepest side at baseline measurements (VDW, 29 mm, ISO 15, Taper.04, Munich, Germany). The defined tip between two marks was cut after the gingival/peri-implant mucosal crevicular fluid was sampled up to the second colored end. Afterwards, the samples were stored in tubes filled with 350 µl Dulbecco’s phosphate buffer saline without CaCl2 and MgCl2 (PBS, Sigma-Aldrich, Steinheim, Germany) + 10% fetal calf serum (FCS, Lonza, Verviers, Belgium) at − 80 °C. After sample assessment, a calibration curve for recovery ELISA standard with paper points was applied. Additionally, the lowest standard parameters for each value were assessed and projected to the dilution of 1:351 with PBS + 10% FCS. All samples were thawed only once for analysis. Cytokines were detected by ELISA as per manufacturer’s instructions. BD Rat ELISA sets were used to measure IL-6 and TNFα (BD Biosciences Pharmingen, San Diego, CA, USA). ELISA was quantified on TECAN Spark 10 M (TECAN AG, Männedorf, Switzerland). The final cytokine concentrations were calculated by multiplication with the dilution factor (× 351) in the storage buffer and the defined recovery coefficient (× 8.52 for IL-6 and × 1.79 for TNF). Detection limits were defined in the same manner using the lowest positive standard of the respective ELISA (0.078 ng/ml for IL-6 and 0.031 ng/ml for TNF).
Clinical parameters included the pocket probing depth (PPD) in mm from the bottom of the sulcus to the gingival/mucosal margin using a Michigan periodontal probe at four points around each unit (Fig. 2A, at sessions 1, 2, and 3, 1 week, 8 weeks, and 12 weeks after surgery)32,33,34. Additionally, the sulcus bleeding index (SBI, Saxer & Mühlemann, score 0 = no bleeding, 1 = isolated bleeding, 2 = confluent linear bleeding, and 3 = severe bleeding) was investigated (sessions 1, 2, and 3). The survival rate showed whether the implant was still present in the animal’s mouth at the time of examination. Implant success was based on the criteria of Jahn and d'Hoedt35. The various success parameters were no implant mobility (in case of any implant mobility, it was considered not successful), no clinical infection (such as gingival inflammation, swelling, secretion and redness of the tissue) or bleeding on probing less than score 2 (0 = no bleeding, 1 = isolated bleeding, 2 = confluent linear bleeding, and 3 = severe bleeding), or increased PPD (the sulcus depths must not exceed 2 mm for 2 consecutive controls)..
In vitro immunological examination
IL-6 and TNFα levels were evaluated after stimulation of rat spleen cells with different materials. After finalization of the animals, 18 rat spleens of the control group were directly prepared and stored in RPMI 1640 without L-Glutamine (Sigma-Aldrich, Steinheim, Germany) with 10% FCS (see above), 2 mM L-Glutamine (Sigma-Aldrich, Steinheim, Germany), 100 U/ml Penicillin (Sigma-Aldrich, Steinheim, Germany) and 100 µg/ml Streptomycin (Sigma-Aldrich, Steinheim, Germany). The rat spleen of one rat without any cellular contact with titanium or zirconia was used as a control group. Using a cell strainer splenocytes were separated from the connecting tissue. Then, erythrocytes were removed by lysis and the remaining leukocytes were used for in vitro experiments. Using spleen cells will give a test for the immunogenicity of the material for proving the tolerance against the foreign material. The in vitro stimulation tests were performed with 2 × 106 rat spleen cells/ml in a 24-well plate (Corning, Amsterdam, Netherlands) and incubated at 37 °C and with 5% CO2 for 3 and 24 h. The stimulation levels of the rat spleen cells for IL-6 and TNFα were assessed for four different materials. These included phytohemagglutine (PHA, PAN-Biotech GmbH, Aidenbach, Germany), lipopolysaccharide (LPS, Sigma-Aldrich Chemie GmbH, Munich, Germany), titaniumoxide nanopowder (Titanium(IV)oxide nanopowder, < 100 nm particle size, Sigma-Aldrich Chemie GmbH, Munich, Germany. and zirconium oxide nanopowder (Zirconium(IV)oxide nanopowder, < 100 nm particle size, Sigma-Aldrich Chemie GmbH, Munich, Germany). The following sample quantities were used (PHA: 2,5 µg/ml, LPS: 250 ng/ml, Titanium Oxide: 100 mg/ml, Zirconium Oxide: 100 mg/ml). Stimulation levels were measured after 3 and 24 h with BD Rat ELISA Sets for IL-6 and TNF (BD Biosciences Pharmingen, San Diego, CA, USA).
Statistical analysis
The sample size was calculated using the nQuery Advisor software (Statsols, Version 8, Cork, Ireland) with McNemar’s test on the equality of paired samples. Using a p = 0.05 significant level, an odds ratio of 0.1530, and a power of 80%36, a group comparison of the target main study parameter produced a sample size of N = 18 rats per group, including two dropouts.
Analyses were performed using Prism 8 software for Mac OS X (GraphPad, La Jolla, CA, USA) running on Apple OS X. The analysis values were tested for normal distribution using the Kolmogorov–Smirnov normality test. Data for immunological parameters over time was analyzed using a mixed-effects model with the Geisser-Greenhouse correction. Furthermore, the Tukey’s multiple comparisons test and the intra-group comparison was assessed with the Kruskal–Wallis test and Dunn’s multiple comparisons test. Values of hypersensitivity reactions were evaluated using a two-way analysis of variance (ANOVA) and a Sidak’s multiple comparison test. P-values < 0.05 were considered statistically significant.
Approval for animal experiments
For experiments involving live vertebrates and/or higher invertebrates, your Methods section must include a statement that:
-
Identifies the institutional and/or licensing committee that approved the experiments, including any relevant details.
-
Confirms that all experiments were performed in accordance with relevant named guidelines and regulations.
-
We confirm that all experiments were performed in accordance with relevant named guidelines and regulations and the study complied with the ARRIVE guidelines.
Results
In vivo immunological and clinical examination
Of 54 rats, 52 were included in the evaluation. Two animals from the group zoledronic acid were lost, one in the course of the drug administration in the rat restrainer and the other in the anesthesia operation, probably to respiratory arrest.
Inter-group analysis around titanium implants regarding IL-6 demonstrated significantly higher values at session 1 (one week after surgery) in the control group when compared to the zoledronic acid group (Fig. 3A, p = 0.02). However, the natural tooth in the control group at session 2 (8 weeks after surgery) presented higher values when compared to the alendronic acid group (p = 0.01). The lowest standard parameter (detection limit) was assessed for IL-6 at 233 (ng/ml) and for TNFα at 19.6 (ng/ml). Within each group over time between sessions 1 and 3 (1 and 12 weeks after surgery) around zirconia implants (p = 0.03) and between sessions 1 and 2 (one and 8 weeks after surgery) around teeth (p = 0.04) with alendronic acid drug administration, a significant reduction of IL-6 was observed (Fig. 3A). Furthermore, comparisons of subgroups over time showed a significant difference at session 2 (8 weeks after surgery) between the titanium implants of the zoledronic acid group and the teeth of the control group (p = 0.03).
The evaluation for interleukin-6 and TNFα levels of both implant materials and the natural tooth were presented. The collection took place at three sessions: 1 week, 8 weeks, and 12 weeks after surgery. The detection limit was assessed for IL-6 at 233 ng/ml and for TNFα at 19.6 ng/ml after correction with dilution and recovery factor.
Inter- and intra-group analysis for TNFα values presented no significant differences within each subgroup, such as with or without drug administration, for both implant materials and the natural teeth (Fig. 3B, p > 0.05).
After 3 months of follow-up, for titanium implants in the zoledronic acid group, a survival rate of 93.00% with a success rate of 60.00% was observed. For the alendronic acid group, it was a survival rate of 55.60% with a success rate of 70.00%, and in the control group without drug administration, it was a survival rate of 37.00% with a success rate of 100% (Table 1, Fig. 4). On the other hand, zirconia implants demonstrated in the zoledronic acid group a survival rate of 75.00% with a success rate of 41.60%; in the alendronic acid group, a survival rate of 55.60% with a success rate of 54.00%; and in the control group without drug administration, a survival rate of 61.20% with a success rate of 72.72%.
Regarding the PPD around teeth in groups with and without drug application, mean values of approximately 1 mm were comparable (Table 2). However, around both implant materials, PPD values were measured at approximately 2 mm in both test groups. Mean SBI value over all subgroups and implant materials and natural teeth demonstrated low inflammation below 0.5 (score between 0 = no bleeding and 1 = isolated bleeding).
In vitro immunological examination
Between the IL-6 measurements at 3 and 24 h time points after stimulation, only LPS stimulation presented a significant IL-6 value increase. When compared to all other groups (control, PHA, titaniumoxide powder, and zirconiumoxide powder), only intra-material analysis 24 h after LPS stimulation of the rat spleen cells revealed a significantly higher IL-6 parameter (Fig. 5A, p < 0.01). TNFα values were comparable to those of IL-6, as LPS stimulation significantly increased TNFα levels between 3 and 24 h (p < 0.01). Additionally, intra-material analysis at 3 h and again at 24 h showed a significantly higher TNFα production after LPS stimulation (Fig. 5B, p < 0.01). The lowest standard parameter (detection limit) was assessed for IL-6 at 0.078 ng/ml and for TNFα at 0.031 ng/ml.
The stimulation levels of the rat spleen cells for interleukin-6 and TNFα were shown for phytohemagglutinine (PHA), lipopolysaccharide (LPS), titaniumoxid-nanopowder and zirconiumoxide nanopowder in relation to a control group. Stimulation levels were assessed after 3 and 24 h. The lowest standard parameter (detection limit) was assessed for IL-6 at 0.078 ng/ml and for TNFα at 0.031 ng/ml.
Discussion
The aim of this rat study was to evaluate the host-inflammatory response of peri-implant interfaces around titanium and zirconia implants in a high-risk group with systematic antiresorptive bisphosphonate drug administrations. Evidence showed that IL-6 polymorphisms were involved in soft tissue inflammation37,38. Furthermore, the significantly higher levels of TNFα in peri-implantitis patients indicated that TNFα also played a key role in peri-implantitis and that TNFα was a proinflammatory cytokine that promoted the resorption of the bone and mediates the infection's inflammatory response27. In this experiment, we sought to determine how the chosen drug administrations and the different materials affected these inflammatory parameters. Our results indicated that IL-6 demonstrated significantly higher values in the control groups without drug administration around titanium implants and the natural tooth. A possible explanation for the increased IL-6 release in the healthy control group could possibly be a better and faster reacting bone metabolism and thus more pronounced inflammatory reaction to a high-risk implant procedure. However, analysis for TNFα values presented no significant differences within each subgroup. Furthermore, in an in vivo study in humans, titanium and zirconia implants performed better than their natural counterparts in terms of IL-6 and TNFα25. By contrast, another mucositis study suggested that the implant group had a significantly higher expression of IL-6 than the healthy tooth group39.
With regard to TNFα, published data are quite controversial. On the one hand, the key role of TNFα in peri-implantitis was indicated by significantly higher levels of TNFα in patients with peri-implantitis compared to those in the control group27. Other analyses found that the risk of dental peri-implant disease was not significantly associated with TNFα-polymorphism40,41.
In terms of survival, the control group had the lowest and the zoledronic group the highest survival rates. However, the success rate was reversed, with the control group having the highest success rate and the zoledronic group the lowest. Data from seven studies enabled comparing the survival rate of titanium implants in a meta-analysis42. The survival rates up to 12 months after loading were very high (> 98%). Similarly, for the material titanium, a current meta-analysis estimated the 1- and 2-year survival rates for dental zirconia implants inserted in humans to be 98.3% and 97.2%, respectively43. These findings agree with the study of Adanez et al., which suggested that the mean survival rate of zirconia implants was 95%44. Intravenous administration, as applied in this study, is associated with a higher risk of peri-implant inflammation and might be the reason for poor success rates45. A possible cause for the reduced success could be the drug reduced bone remodeling3. It is evident that bisphosphonates inhibit osteoclasts and thus negatively affect not only the blood supply to the peri-implant bone but also the healing capacity3,7,8. In the case of inflammation, therefore, a reduced blood supply and immune defense would have to be assumed. Further studies should include a follow-up longer than 3 months, as it should be assumed that the bisphosphonate groups may demonstrate higher implant loss rates due to the adverse success rates.
Besides the in vivo immunological findings of this study, the stimulation effect of titanium and zirconia nanoparticles on host inflammatory parameters was also assessed in an in vitro set-up. Patients may be exposed internally to nanoparticles by wear mechanisms associated with dental implants46. When macrophages phagocytose these nanoparticles, they release many pro-inflammatory and pro-osteoclastogenic cytokines, such as TNFα and IL-6, to promote osteoclastogenesis46. Wear is not expected directly after insertion but low product quality may be a reason for particle release after implant insertion47,48. If released particles affect implant performance is not clear yet. Zhang et al. demonstrated that zirconia nanoparticles were bioactive to cells, and their results indicated that such nanoparticles might be more irritating to macrophages than titanium microparticles49. In addition to the effects of zirconia nanoparticles, effects of titanium particles have also been described50. Although, like zirconia, the block biocompatibility properties of titanium are excellent51, the adverse effects of titanium particles on osteoblast function have been noted52. Our in vitro stimulation tests suggested the biocompatibility of both titanium and zirconia nanoparticles with rat cells. Furthermore, He et al. revealed that titanium and zirconia dental implants demonstrated titanium and zirconia concentrations in the bone tissue of porcine jaws53. The titanium content released by titanium implants was twice as high as the zirconia content released by zirconia implants53. In addition, zirconia nanoparticles presented lower cytotoxicity and DNA damage compared to the results reported for titanium nanoparticles in human cells. The current data situation regarding the exact chemical composition of nanoparticles in peri-implant tissue (either zirconia or titanium particles) is currently unclear. Based on conventional elemental analysis, it is currently not possible to say in which chemical form the nanoparticles are actually present in the tissue (oxidized, ionized, elemental). This should be considered when interpreting the data”.
A limitation of this study was that the split-mouth design was lost in several animals, as they lost one or more implants. Additionally, we recognize that IL-6 and TNFα metabolism can vary between our rat model and humans. For future studies immunohistochemical results for the tissue-implant-interface are a very interesting study design approach, as local presence of IL-6 and TNF-a within the tissue-implant interface may differ between paper point collection levels. When interpreting the results, it should be noted that the soft- and not the hard tissue was analyzed.
Conclusions
Regarding in vivo and in vitro IL-6 and TNFα production, no implant material was superior to the other. No significant in vitro stimulation of rat spleen cells was detected with respect to titanium oxide and zirconium oxide nanoparticles. Systemic bisphosphonate delivery led to decreased implant success after up to 3 months of follow-up. However, in terms of implant survival, the control group had the lowest rate and the zoledronic group the highest. According to our data no better prognosis could be achieved with antiresorptive therapy using alternative implant materials than with standard titanium implants. Therefore, the indication cannot be shifted more clearly in favor of implant placement.
References
Then, C. et al. Incidence and risk factors of bisphosphonate-related osteonecrosis of the jaw in multiple myeloma patients having undergone autologous stem cell transplantation. Onkologie 35, 658–664. https://doi.org/10.1159/000343950 (2012).
Nagy, V. & Penninger, J. M. The RANKL-RANK Story. Gerontology 61, 534–542. https://doi.org/10.1159/000371845 (2015).
Dlamini, N. L., Mukaya, H. E., Van Zyl, R. L., Jansen van Vuuren, N. C. & Mbianda, X. Y. Carbon nanospheres conjugated bisphosphonates: synthesis, characterization and in vitro antimalarial activity. Artif. Cells Nanomed. Biotechnol. 46, 287–296. https://doi.org/10.1080/21691401.2018.1491481 (2018).
Drake, M. T., Clarke, B. L. & Khosla, S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin Proc. 83, 1032–1045. https://doi.org/10.4065/83.9.1032 (2008).
Aljohani, S. et al. What is the effect of anti-resorptive drugs (ARDs) on the development of medication-related osteonecrosis of the jaw (MRONJ) in osteoporosis patients: A systematic review. J. Cranio-maxillofac. Surg. 45, 1493–1502. https://doi.org/10.1016/j.jcms.2017.05.028 (2017).
Allen, M. R. & Burr, D. B. The pathogenesis of bisphosphonate-related osteonecrosis of the jaw: so many hypotheses, so few data. J. Oral Maxillof. Surg. 67, 61–70. https://doi.org/10.1016/j.joms.2009.01.007 (2009).
Walter, C. et al. Influence of bisphosphonates on endothelial cells, fibroblasts, and osteogenic cells. Clin. Oral Investig. 14, 35–41. https://doi.org/10.1007/s00784-009-0266-4 (2010).
Ziebart, T. et al. Bisphosphonates: restrictions for vasculogenesis and angiogenesis: inhibition of cell function of endothelial progenitor cells and mature endothelial cells in vitro. Clin. Oral Investig. 15, 105–111. https://doi.org/10.1007/s00784-009-0365-2 (2011).
Pabst, A. M. et al. The influence of bisphosphonates on viability, migration, and apoptosis of human oral keratinocytes–in vitro study. Clin. Oral Investig. 16, 87–93. https://doi.org/10.1007/s00784-010-0507-6 (2012).
Henry, D. H. et al. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J. Clin. Oncol. 29, 1125–1132. https://doi.org/10.1200/jco.2010.31.3304 (2011).
Guarneri, V. et al. Bevacizumab and osteonecrosis of the jaw: incidence and association with bisphosphonate therapy in three large prospective trials in advanced breast cancer. Breast Cancer Res. Treat. 122, 181–188. https://doi.org/10.1007/s10549-010-0866-3 (2010).
Holzinger, D. et al. Effect of dental implants on bisphosphonate-related osteonecrosis of the jaws. J. Oral Maxillofac. Surg. 72(1937), e1931-1938. https://doi.org/10.1016/j.joms.2014.04.037 (2014).
Jacobsen, C. et al. Osteopathology induced by bisphosphonates and dental implants: clinical observations. Clin. Oral Investig. 17, 167–175. https://doi.org/10.1007/s00784-012-0708-2 (2013).
Kwon, T. G. et al. Osteonecrosis associated with dental implants in patients undergoing bisphosphonate treatment. Clin. Oral Implant Res. 25, 632–640. https://doi.org/10.1111/clr.12088 (2014).
Lopez-Cedrun, J. L. et al. Oral bisphosphonate-related osteonecrosis of the jaws in dental implant patients: a case series. Br. J. Oral Maxillofac. Surg. 51, 874–879. https://doi.org/10.1016/j.bjoms.2013.06.011 (2013).
Tam, Y., Kar, K., Nowzari, H., Cha, H. S. & Ahn, K. M. Osteonecrosis of the jaw after implant surgery in patients treated with bisphosphonates–a presentation of six consecutive cases. Clin. Implant Dent. Relat. Res. 16, 751–761. https://doi.org/10.1111/cid.12048 (2014).
Al-Sabbagh, M., Robinson, F. G., Romanos, G. & Thomas, M. V. Osteoporosis and bisphosphonate-related osteonecrosis in a dental school implant patient population. Implant Dent 24, 328–332. https://doi.org/10.1097/id.0000000000000234 (2015).
Bell, B. M. & Bell, R. E. Oral bisphosphonates and dental implants: A retrospective study. J. Oral Maxillofac. Surg. 66, 1022–1024. https://doi.org/10.1016/j.joms.2007.12.040 (2008).
Famili, P., Quigley, S. & Mosher, T. Survival of dental implants among post-menopausal female dental school patients taking oral bisphosphonates: a retrospective study. Compend. Contin. Educ. Dent. 32, E106-109 (2011).
Goss, A., Bartold, M., Sambrook, P. & Hawker, P. The nature and frequency of bisphosphonate-associated osteonecrosis of the jaws in dental implant patients: A South Australian case series. J. Oral Maxillofac. Surg. 68, 337–343. https://doi.org/10.1016/j.joms.2009.09.037 (2010).
Memon, S., Weltman, R. L. & Katancik, J. A. Oral bisphosphonates: early endosseous dental implant success and crestal bone changes. A retrospective study. Int. J. Oral Maxillofac. Implants 27, 1216–1222 (2012).
Hasegawa, Y. et al. Influence of dentures in the initial occurrence site on the prognosis of bisphosphonate-related osteonecrosis of the jaws: a retrospective study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 114, 318–324. https://doi.org/10.1016/j.oooo.2012.04.002 (2012).
Jabbour, Z., El-Hakim, M., Mesbah-Ardakani, P., Henderson, J. E. & Albuquerque, R. Jr. The outcomes of conservative and surgical treatment of stage 2 bisphosphonate-related osteonecrosis of the jaws: a case series. Int. J. Oral Maxillofac. Surg. 41, 1404–1409. https://doi.org/10.1016/j.ijom.2012.05.012 (2012).
Vahtsevanos, K. et al. Longitudinal cohort study of risk factors in cancer patients of bisphosphonate-related osteonecrosis of the jaw. J. Clin. Oncol. 27, 5356–5362. https://doi.org/10.1200/jco.2009.21.9584 (2009).
Clever, K. et al. Experimental peri-implant mucositis around titanium and zirconia implants in comparison to a natural tooth: part 1-host-derived immunological parameters. Int. J. Oral Maxillofac. Surg. 48, 554–559. https://doi.org/10.1016/j.ijom.2018.10.018 (2019).
Clever, K. et al. Experimental peri-implant mucositis around titanium and zirconia implants in comparison to a natural tooth: part 2-clinical and microbiological parameters. Int. J. Oral Maxillofac. Surg. 48, 560–565. https://doi.org/10.1016/j.ijom.2018.10.017 (2019).
Darabi, E., Kadkhoda, Z. & Amirzargar, A. Comparison of the levels of tumor necrosis factor-alpha and interleukin-17 in gingival crevicular fluid of patients with peri-implantitis and a control group with healthy implants. Iran J. Allergy Asthma Immunol. 12, 75–80 (2013).
Guncu, G. N., Akman, A. C., Gunday, S., Yamalik, N. & Berker, E. Effect of inflammation on cytokine levels and bone remodelling markers in peri-implant sulcus fluid: a preliminary report. Cytokine 59, 313–316. https://doi.org/10.1016/j.cyto.2012.04.024 (2012).
Ramseier, C. A. et al. Host-derived biomarkers at teeth and implants in partially edentulous patients. A 10-year retrospective study. Clin. Oral Implants Res. 27, 211–217. https://doi.org/10.1111/clr.12566 (2016).
Abtahi, J., Agholme, F., Sandberg, O. & Aspenberg, P. Effect of local vs. systemic bisphosphonate delivery on dental implant fixation in a model of osteonecrosis of the jaw. J. Dent. Res. 92, 279–283. https://doi.org/10.1177/0022034512472335 (2013).
Bernhardsson, M., Sandberg, O. & Aspenberg, P. Anti-RANKL treatment improves screw fixation in cancellous bone in rats. Injury 46, 990–995. https://doi.org/10.1016/j.injury.2015.02.011 (2015).
Mombelli, A., van Oosten, M. A., Schurch, E. Jr. & Land, N. P. The microbiota associated with successful or failing osseointegrated titanium implants. Oral Microbiol. Immunol. 2, 145–151 (1987).
Loe, H. The Gingival Index, the Plaque Index and the Retention Index Systems. J. Periodontol. 38(Suppl), 610–616. https://doi.org/10.1902/jop.1967.38.6.610 (1967).
Saxer, U. P. & Muhlemann, H. R. Motivation and education. SSO Schweiz Monatsschr Zahnheilkd 85, 905–919 (1975).
Jahn, M. & d’Hoedt, B. Zur Definition des Erfolges bei dentalen Implantaten. Zahnärztl Implantol 8, 221–226 (1992).
Faul, F., Erdfelder, E., Buchner, A. & Lang, A. G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 41, 1149–1160. https://doi.org/10.3758/BRM.41.4.1149 (2009).
Severino, V. O., Napimoga, M. H. & de Lima Pereira, S. A. Expression of IL-6, IL-10, IL-17 and IL-8 in the peri-implant crevicular fluid of patients with peri-implantitis. Arch. Oral Biol. 56, 823–828. https://doi.org/10.1016/j.archoralbio.2011.01.006 (2011).
Scapoli, L. et al. Interleukin-6 gene polymorphism modulates the risk of periodontal diseases. J. Biol. Regul. Homeost Agents 29, 111–116 (2015).
Ata-Ali, J., Flichy-Fernandez, A. J., Ata-Ali, F., Penarrocha-Diago, M. & Penarrocha-Diago, M. Clinical, microbiologic, and host response characteristics in patients with peri-implant mucositis. Int. J. Oral Maxillofac. Implants 28, 883–890. https://doi.org/10.11607/jomi.2654 (2013).
Mo, Y. Y. et al. Association between tumor necrosis factor-alpha G-308A polymorphism and dental peri-implant disease risk: A meta-analysis. Medicine (Baltimore) 95, e4425. https://doi.org/10.1097/md.0000000000004425 (2016).
Cury, P. R. et al. Effect of tumor necrosis factor-alpha gene polymorphism on peri-implant bone loss following prosthetic reconstruction. Implant Dent. 16, 80–88. https://doi.org/10.1097/ID.0b013e31803277c1 (2007).
Tattan, M., Chambrone, L., González-Martín, O. & Avila-Ortiz, G. Static computer-aided, partially guided, and free-handed implant placement: A systematic review and meta-analysis of randomized controlled trials. Clin. Oral Implant Res. 31, 889–916. https://doi.org/10.1111/clr.13635 (2020).
Roehling, S., Schlegel, K. A., Woelfler, H. & Gahlert, M. Performance and outcome of zirconia dental implants in clinical studies: A meta-analysis. Clin. Oral Implant Res. 29(Suppl 16), 135–153. https://doi.org/10.1111/clr.13352 (2018).
HaroAdanez, M., Nishihara, H. & Att, W. A systematic review and meta-analysis on the clinical outcome of zirconia implant-restoration complex. J. Prosthodont. Res. 62, 397–406. https://doi.org/10.1016/j.jpor.2018.04.007 (2018).
Walter, C., Al-Nawas, B., Wolff, T., Schiegnitz, E. & Grötz, K. A. Dental implants in patients treated with antiresorptive medication—A systematic literature review. Int. J. Implant Dent. 2, 9. https://doi.org/10.1186/s40729-016-0041-7 (2016).
Ingham, E. & Fisher, J. The role of macrophages in osteolysis of total joint replacement. Biomaterials 26, 1271–1286. https://doi.org/10.1016/j.biomaterials.2004.04.035 (2005).
El Hassanin, A. et al. Effect of implant surface roughness and macro- and micro-structural composition on wear and metal particles released. Materials (Basel) 14, 6800. https://doi.org/10.3390/ma14226800 (2021).
Silva, G. A. F., Faot, F., Possebon, A., da Silva, W. J. & Del BelCury, A. A. Effect of macrogeometry and bone type on insertion torque, primary stability, surface topography damage and titanium release of dental implants during surgical insertion into artificial bone. J. Mech. Behav. Biomed. Mater. 119, 104515. https://doi.org/10.1016/j.jmbbm.2021.104515 (2021).
Zhang, Y. F., Zheng, Y. F. & Qin, L. A comprehensive biological evaluation of ceramic nanoparticles as wear debris. Nanomedicine 7, 975–982. https://doi.org/10.1016/j.nano.2011.04.005 (2011).
Choi, M. G. et al. Effects of titanium particle size on osteoblast functions in vitro and in vivo. Proc. Natl. Acad. Sci. U. S. A. 102, 4578–4583. https://doi.org/10.1073/pnas.0500693102 (2005).
Nugiel, D. J., Wood, D. J. & Sung, K. L. Quantification of adhesiveness of osteoblasts to titanium surfaces in vitro by the micropipette aspiration technique. Tissue Eng. 2, 127–140. https://doi.org/10.1089/ten.1996.2.127 (1996).
Lombardi, A. V. Jr., Mallory, T. H., Vaughn, B. K. & Drouillard, P. Aseptic loosening in total hip arthroplasty secondary to osteolysis induced by wear debris from titanium-alloy modular femoral heads. J. Bone Joint Surg. Am. 71, 1337–1342 (1989).
He, X. et al. Titanium and zirconium release from titanium- and zirconia implants in mini pig maxillae and their toxicity in vitro. Dent. Mater. 36, 402–412. https://doi.org/10.1016/j.dental.2020.01.013 (2020).
Acknowledgements
The authors thank the Institute Straumann AG for the fabrication of dental implants and also thank Birgit Plümäkers (Institute of Immunology, University Hospital RWTH Aachen, Pauwelsstraße 30, Germany) for excellent technical assistance. Furthermore, the authors report no conflicts of interest related to this study.
Funding
Open Access funding enabled and organized by Projekt DEAL. This study was funded by the START funding program from the medical faculty of Aachen University. It was part of research Project 105/18.
Author information
Authors and Affiliations
Contributions
K.K.: conception and design, acquisition of data, drafting the work, final approval; L.R.: conception and design, revising the work, final approval; J.W.: analysis and interpretation, revising the work, final approval; S.M.: acquisition of data, revising the work, final approval; F.P.: analysis and interpretation, revising the work, final approval; MH: conception and design, revising the work, final approval; F.H.: analysis and interpretation, revising the work, final approval; A.M.: conception and design, analysis and interpretation, revising the work, final approval; Each author participated sufficiently in the work to take public responsibility for appropriate portions of the content; Each author agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kniha, K., Rink, L., Wolf, J. et al. Host inflammatory response and clinical parameters around implants in a rat model using systemic alendronate and zoledronate acid drug administrations. Sci Rep 12, 4431 (2022). https://doi.org/10.1038/s41598-022-08308-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-08308-8
- Springer Nature Limited