Abstract
Background
Macular hole (MH) is a rare complication of retinitis pigmentosa (RP) and has an adverse impact on residual visual function. At present, the underlying mechanisms are not fully understood and surgical experience is limited.
Methods
We reviewed the medical records and optical coherence tomography (OCT) scans in a cohort of eight eyes of seven RP patients with MH in order to report their OCT features and vitreoretinal surgical prognosis.
Results
This study includes four lamellar macular holes (LMHs) and four full-thickness macular holes (FTMHs). Pre-operative OCT revealed other macular abnormalities in all eyes, such as epiretinal membrane (ERM), cystoid macular edema (CME), lamellar hole-associated epiretinal proliferation (LHEP) and vitreoretinal traction. MH progression and subjective vision worsening were noted in one LMH eye during a seven-month follow-up. All holes closed after vitrectomy with internal limiting membrane (ILM) peeling. At final follow-up, one eye had improved vision and seven eyes remained stable compared to baseline.
Conclusions
The occurrence of MH in RP is accompanied by various imaging characteristics, such as ERM, CME and LHEP, suggesting a multifactorial pathogenesis. Considering poor vision in most RP patients with potentially progressive MH, surgery appears to be effective in maintaining or improving the central vision in a period of time. Thus, vitrectomy should be performed as soon as possible and flap-assisted techniques or episcleral surgeries are needed for some special cases.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Retinitis pigmentosa (RP) encompasses a set of complex hereditary retinal diseases characterized by progressive degeneration of rod and cone photoreceptors. The worldwide prevalence of RP is about 1 in 4000 with well over 100 genes implicated so far [1, 2]. The clinical symptoms of RP manifest a classical order, usually night blindness is the first symptom, followed by the progressive loss of the peripheral visual field [3]. In the end stage, only a small residual central island of the visual field may remain, which results in severely constricted vision known clinically as ‘tunnel vision’ [4]. However, macular abnormalities are relatively frequent among RP patients, leading to additional visual disturbances, such as macular atrophy, choroidal neovascularization, cystoid macular edema (CME) and vitreomacular interface disorders. Compared to others, macular hole (MH) is a rare complication of RP with a reported prevalence of 0.5 − 4.5% [5, 6]. Earlier studies have shown several clinical findings indicating the possible pathogenesis of RP with MH [7, 8]. More clinical features of these eyes remain to be seen, and high-resolution spectral-domain optical coherence tomography (SD-OCT) can help us understand them better.
Vitrectomy has rarely been reported for RP patients with MH, although it is widely used for MH repair in general patients. Due to the limited experience, it is important to study the outcomes and prognosis of vitreoretinal surgical treatment. Thus, our study aimed to describe the clinical features and surgical intervention outcomes of RP patients with MH.
Methods
Our retrospective review enrolled the records of seven RP patients with MH treated by vitrectomy. This study was followed the principles outlined in the Declaration of Helsinki. Approval was obtained by the Medical Ethics Committee of Qilu Hospital of Shandong University, and informed consent was obtained from all patients. Macular hole surgery was performed in eight eyes by two experienced surgeons using a standard 23- or 25-gauge three-port pars plana vitrectomy (PPV). Complete removal of the posterior hyaloid was performed in all eyes with or without the use of triamcinolone acetonide. Then about 0.2 mL ICG-diluted solution (0.25%) was gently injected into the vitreous cavity and immediately washed out with balanced solution, and the stained ILM was removed with a forceps. Atypical epiretinal proliferation, also referred to as lamellar hole-associated epiretinal proliferation (LHEP), was identified as a sticky epiretinal membrane with yellowish pigment. LHEP was also carefully removed while the parts tightly attached to the edge of the hole margin was left behind to prevent unnecessary loss of normal tissue.Two eyes experienced free ILM flap insertion into the macular hole. All surgeries ended with air-fluid exchange or silicone oil tamponade. If necessary, a concomitant cataract operation was performed.
Results
Our study included seven patients (male: 2, female:5), the mean age was 49.3 ± 13.7 years, and one patient had a bilateral presentation. All patients complained of a recent reduction in visual acuity in their affected eyes, with or without metamorphopsia. Preoperative best-corrected visual acuity (BCVA) ranged from hand motion vision to 20/50. With dilated fundoscopy, all eyes presented typical signs of RP, including peripheral retinal atrophy with bone spicule-shaped pigmentation in the mid-periphery, waxy pallor of the optic nerve head and attenuation of retinal vessels.
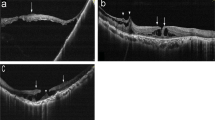
By SD-OCT, four eyes were diagnosed with lamellar macular hole (LMH) and four with full-thickness macular hole (FTMH) (Fig. 1 and Table 1). Cases No. 1 and 7 had the presence of a foveal bump (Fig. 1a, g), and they had other similar features such as atypical epiretinal proliferation and outer retinal layer defection, both more severe in the temporal retina. Only two eyes (cases No. 2 and 3) showed posterior vitreous detachment (PVD), of which one showed incomplete PVD with apparent vitreoretinal traction (Fig. 1b) and the other one was high myopia exhibiting macular retinal detachment with complete PVD, posterior staphyloma and retinoschisis (Fig. 1c). Interestingly, they were the only two eyes who showed no atypical epiretinal proliferation. And unlike the small interretinal cystoid spaces in others, case No.2 presented a larger extent of a CME. We suspect that the formation of large cystoid spaces are related to vitreoretinal traction, and atypical epiretinal proliferation tends not to be present when mechanical retinal traction plays a dominant role in MH formation. Cases No. 4, 5 and 6 showed similar OCT images, especially atypical epiretinal proliferation at the hole margin. However, compared to case No. 4 with small FTMH and case No. 5 with LMH, case No. 6 had the largest size of the macular hole 720 μm in diameter. To some extent, these may indicate different periods of MH development and inform us that this disease is often progressive rather than dormant.
Images a-g and h-n respectively corresponded to the pre- and post-operative images of seven patients in Table 1, excluding the right eye of case No. 6. a-g The pre-operative horizontal OCT images of seven surgical eyes. Notice the epiretinal proliferation (arrow) that isoreflective and conformed to the retinal surface. The asterisk pointed to a presence of foveal bump. h-n The post-operative horizontal OCT images showed the MHs were closed
Besides, all eyes showed the atrophy of the outer retinal layers and the macula was usually steep, especially in myopic cases (Fig. 1a, c and d). Epiretinal membrane (ERM) was noted in six eyes, although not significantly. CME was noted in five eyes. Contrary to previous reports [9], most cystoid spaces were present in the outer nuclear layer. In fact, the distribution of CME and LHEP was asymmetric in the temporal and nasal retina, and there was a corresponding relationship between the two in location (Fig. 2a, b).
In the fellow eyes of the patients with unilateral lesions, thinning and disappearing of integrity of the outer retinal layer were also observed. All eyes showed relatively good central vision (20/50 to 20/20) except one high myopic eye whose BCVA was counting fingers. One eye showed incomplete PVD (Fig. 2c). None of the eyes showed CME or LHEP. In one eye whose BCVA was 20/20, OCT demonstrated serious atrophy of the outer retinal layers and a small part of the ellipsoid zone preserved only in the fovea (Fig. 2d). This may indicate that the presence of MH is a huge blow to the residual central vision of RP patients.
Three patients with LMHs (cases No. 1, 5 and 7) and two patients with FTMHs (cases No. 2 and 4) underwent vitrectomy with ILM peeling and air tamponade. Phacoemulsification and posterior chamber intraocular lens implantation were simultaneously performed in cases No. 5 and 7 for cortical cataract and posterior subcapsular cataract, respectively. These patients were advised to maintain the prone position for 3 to 5 days after surgery. Post-operative follow-up showed successful closure of all holes (Fig. 1).
Considering macular hole-related retinal detachment in case No. 3 with high myopia, he accepted vitrectomy, phacoemulsification, and ILM peeling. After covering the base of the hole with the residual part of the trimmed ILM, oil tamponade was conducted. One week later, OCT showed a failure of macular retinal reattachment, and ILM tissue inserted in the hole was observed (Fig. 1j). At 3-month postsurgery, the MH closed while the retina remained detached, despite decreased macular thickness. Due to the poor anatomical and functional results, silicone oil has not been removed so far.
In the left eye of case No. 6 with cortical cataract and a large hole, vitrectomy was performed with concomitant removal of the cataract. Similarly, the trimmed ILM flap was used for inserting the hole and then silicon oil was injected. Ten days after surgery, OCT confirmed successful closure of the hole (Fig. 1m). Silicone oil removal as well as posterior chamber intraocular lens implantation were performed three months after vitrectomy.
For the right eye of case No.6 whose BCVA was 20/80, we initially did not intervene and kept her under regular follow-up. During the seven-month follow-up period, she reported marked subjective visual deterioration, although her BCVA remained unchanged. Of note, the hole showed a clear tendency to progress to full thickness compared to the baseline (Fig. 3). This spontaneous conversion was accompanied by the exacerbation of existing CME, ERM as well as LHEP. Progressive defection of the ellipsoidal zone was also obvious in this process. Finally, the patient underwent the same surgical protocol as cases No. 5 and 7 for LMH repair, which resulted in a good outcome without vision worsening.
a Colour fundus photograph at baseline revealed classical triad of waxy disc pallor, attenuated vessels and bony spicules. b Automated 30–2 Humphrey visual field testing at baseline (mean deviation, − 25.56 decibels [dB]). c SD-OCT at baseline showed a LMH with inner retinal layer disruption, ERM, mild LHEP (asterisk) and CME. d SD-OCT obtained three months later showed a bridge-like structure at macula fovea composed of outer nuclear layer and a small part of photoreceptor inner segments with wide deletion of both inner and outer retinal layers. e SD-OCT obtained seven months later showed the bridge-like structure collapsed and filled in the bottom of the macular hole (arrow). f At 2-week post-surgery, SD-OCT confirmed a successful closure of the macular hole, with CME resolved
At final follow-up (3–12 months postoperatively), one eye had improved vision and seven eyes remained stable compared to the baseline. Almost all patients perceived improvement in visual function. Postoperative OCT showed CME resolved significantly with reduced central retinal thickness. However, outer retinal atrophic changes were detected in all eyes except cases No. 2 and 3, especially the destruction and deletion of the ellipsoidal zone.
Discussion
Currently, the exact etiology behind MH formation in RP remains unclear and a multifactorial pathogenesis is widely proposed. Vitreoretinal interface changes are considered as a major mechanism. Principally, degenerative vitreous with the collapse of the vitreous gel and posterior vitreous detachment may lead to vitreomacular interface changes, which are likely to contribute to the formation of CME [6]. Moreover, both the collapse of the blood-retinal barrier and dysfunction of Müller cells can result in RPE pumping failure, which may eventually provoke intraretinal fluid accumulation and CME development [10]. As a result, rupture and posterior fusion of the cysts may facilitate MH formation [11]. Besides, previous studies showed high myopia and posterior staphyloma can occur in RP eyes [12]. Myopic traction maculopathy, characterized by retinoschisis, macular hole, and foveal retinal detachment, typically contributes to the development of myopic MH [13, 14]. LHEP is an epiretinal tissue with homogenous moderate reflectivity filling the space between the epiretinal membrane and the retinal nerve fiber layer on OCT [15]. It is possible that LHEP without exerting tractional effects, originates from the middle retinal layer because it contains retinal glial cells, specifically Müller cells [16, 17]. The presence of LHEP is said to be associated with more severe macular lesions and outer retinal disruption [18]. LMH eyes with LHEP can occasionally progress to FTMH, and these patients tend to have a failure of FTMH closure [19, 20]. However, the role of LHEP in MH development is poorly understood.
Pars plana vitrectomy (PPV) with ILM peeling stands as a valuable treatment option in the general population with MH [21], but its application in RP remains controversial. To date, only small sample-sized works have been published, and we summarized eighteen previous clinical applications [6, 8, 22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37]. There were a total of 33 patients and the mean age was 46.72 ± 13.50 years (Table 2). Preoperative OCT showed that more than 60% of cases had significant vitreoretinal interface diseases, such as ERM (14%), CME (17.1%), PVD (17.1%), LHEP (14%). The median postoperative follow-up was 6 months (range 1 month to 12 years). Postoperative OCT showed central retinal thickness decreased in eyes with CME. Among the 35 operated eyes, 22(91.4%) eyes had complete closure of the hole, 2 (5.7%) eyes failed to close the hole, and 1(2.9%) eye reopened 2 years later. Of note, five cases with retinal detachment were involved, of which four were accompanied by high myopia. All of them resulted in MH closed with vision improvement. At the last follow-up, 24(68.6%) cases had improved vision, 4(11.4%) remained unchanged, and 7(20%) worsened. In those cases with reduced vision, 3 eyes had progressive severe retinal atrophy, 2 eyes kept MH opened, and 2 had high myopia or foveal detachment.
Previous surgical treatments featured classic ILM peeling and gas or silicon oil tamponade, and flap-assisted techniques were adopted in about a quarter of cases, such as inverted ILM flap or ILM free flap transplantation, LHEP transplantation or autologous lens capsular flap transplantation. About half of the eyes became pseudophakia eventually and the average age was 47.6(28 to 67) years. Two eyes experienced more than one time of PPV and both eventually had worsening vision. In our series, cases with LMH or small FTHM were treated by PPV with conventional ILM peeling and air tamponade. For FTMH eyes with larger hole size or macular hole-related retinal detachment, ILM free flap transplantation was performed to help bridge the hole, fill the gap, act as a scaffold and modulate Müller cell migration and proliferation [38]. Finally, all holes achieved anatomic successful closure after the primary repair. Considering that multiple vitreoretinal surgeries might not be tolerated by RP patients, selecting the best surgical option plays a crucial role in increasing the success rate of primary repair of MH. There are no clear standards for selecting which tamponade agent. The surgeon should take into account multiple factors, such as the number of surgeries, the success rate, the patient's vision needs during the recovery period and so on.
Almost all operated eyes followed for more than 2 years showed retinal thickness decreased and varying degrees of retinal atrophy, of which half were accompanied by progressive vision loss [24, 29, 30]. Despite the short follow-up period, this phenomenon was also obvious in our series. On the basis of retinal atrophy at the macula caused by long-term damage to the photoreceptor or retinal pigment epithelial loss, further retinal thinning after vitrectomy appears to be the main reason for vision deterioration and hole reopening [30]. These atrophic changes may also be secondary to mechanical insult to the retina, phototoxicity by endoillumination, or injury to the retinal pigment epithelium by indocyanine green [31, 39]. Given the natural progression of RP, it is difficult to ascertain the role of surgical trauma in postoperative retinal atrophy. However, from the current perspective, surgery is not without merit, particularly for patients with good vision and eary stage of MH. It may be more meaningful to extend patient follow-up and evaluate the stabilization time of patients' postoperative vision, rather than just the improvement of postoperative vision.
Sieving and Fishman reported a significant myopic shift of the mean spherical refractive error of RP patients from the mean of a normal population [40]. A study of 365 RP patients in Koreans reported that the prevalence of high myopia was up to 16.1% (40 of 249 eyes) [41]. Moreover, Komori and coworkers reported a high incidence of steep macular curvature in non-highly myopic RP eyes, and the steepness was affected by the degree of photoreceptor degeneration [42]. Although the role of high myopia in RP remains to be elucidated, retinal degeneration and visual acuity deterioration are more apparent in patients with high myopia [43]. The occurrence of myopic traction maculopathy further complicates the RP patient's condition. Because of the poor retinal adhesion to the underlying surface and the posterior staphyloma that may produce an inverse traction to impede retinal adhesion, the surgical repair is challenging [44, 45]. Retinal traction cannot be completely eliminated by means of vitreoretinal surgery alone since retinal stretching caused by posterior staphyloma and other components are still present [46]. Just like our high myopic patient with an axial length of 28.2 mm, the retina failed to attach in the short term, despite silicon oil injection. In contrast, Arora et al. combined macular buckling and vitrectomy to treat a myopic macular hole foveal detachment associated with posterior pole staphyloma in a RP eye, which resulted in MH closed with posterior pole flattening [22]. Therefore, to flatten the concavity of the posterior part of the eye and release the inverse traction caused by the posterior staphyloma, episcleral surgeries such as posterior scleral reinforcement and macular buckle are necessary [47].
Spontaneous closure of MH in three eyes was once described [8, 48]. In other cases, MH tends to progress to a larger lesion in the degree and depth of retinal disruption. Based on previous studies, surgical treatment is usually considered beneficial and a successful closure of the hole is achieved in the majority, notwithstanding the long-standing retinal dysfunction as well as the potential risks of vitrectomy. Thus, surgery is worth attempting in order to remain or improve the central vision in some cases, although the ultimate prognosis of these patients may still be poor. We believe that the criteria for assessing the feasibility of surgery should not be limited to improved vision postoperatively, and that stopping the vision loss caused by MH is equally relevant. For the patients themselves, if early surgery allows them to maintain relatively good visual acuity for a certain period of time, this is a good enough indication that they can benefit from the surgery.Several times surgeries are unadvisable, which would lead to undesirable consequences, such as visual acuity worsening and severe retinal atrophy. Careful consideration before vitrectomy is important, and screening RP with OCT is highly recommended to follow-up the patients, evaluate the natural history of the disease as well as identify those patients who could benefit from PPV.
In summary, the occurrence of MH in RP is accompanied by various macular abnormalities, suggesting a multifactorial pathogenesis. In our cases, microincision vitrectomy surgery for RP with MH appears to be effective and safe for helping stabilize the remnant vision for a period of time. Therefore, screening for MH in RP patients is vital, and surgical management should be selected as soon as possible if the hole progresses. However, not all surgeons are suitable to try this treatment as it requires extensive experience and subtle manipulation, sometimes referrals are advisable. Surgeons must be very caution to decide whether or not to indicate surgery for such cases, due to the potential risks that might outweigh the potential benefits for these already poor prognosis patients.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- MH:
-
Macular hole
- RP:
-
Retinitis pigmentosa
- OCT:
-
Optical coherence tomography
- LMH:
-
Lamellar macular hole
- FTMH:
-
Full-thickness macular hole
- PPV:
-
Pars plana vitrectomy
- ILM:
-
Internal limiting membrane
- LHEP:
-
Lamellar hole-associated epiretinal proliferation
- SD-OCT:
-
Spectral-domain optical coherence tomography
- PVD:
-
Posterior vitreous detachment
- CME:
-
Cystoid macular edema
- ERM:
-
Epiretinal membrane
- BCVA:
-
Best corrected visual acuity
References
Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet. 2006;368(9549):1795–809. https://doi.org/10.1016/s0140-6736(06)69740-7.
Nguyen XT, Moekotte L, Plomp AS, Bergen AA, van Genderen MM, Boon CJF. Retinitis pigmentosa: current clinical management and emerging therapies. Int J Mol Sci. 2023; 24(8). https://doi.org/10.3390/ijms24087481.
Hamel C. Retinitis pigmentosa. Orphanet J Rare Dis. 2006;1:40. https://doi.org/10.3390/ijms24087481.
Xu M, Zhai Y, MacDonald IM. Visual Field Progression in Retinitis Pigmentosa. Invest Ophthalmol Vis Sci. 2020;61(6):56. https://doi.org/10.1167/iovs.61.6.56.
Testa F, Rossi S, Colucci R, Gallo B, Di Iorio V, della Corte M, Azzolini C, Melillo P, Simonelli F,. Macular abnormalities in Italian patients with retinitis pigmentosa. Br J Ophthalmol. 2014;98(7):946–50. https://doi.org/10.1136/bjophthalmol-2013-304082.
Lee CY, Yang CM, Yang CH, Hu FR, Chen TC. Flap technique-assisted surgeries for advanced retinitis pigmentosa complicated with macular hole: a case report and literature review. BMC Ophthalmol. 2021;21(1):322. https://doi.org/10.1186/s12886-021-02082-3.
Antropoli A, Arrigo A, Bianco L, Cavallari E, Berni A, Casoni F, Consalez G, Bandello F, Cremona O, Battaglia Parodi M. HYPERREFLECTIVE BAND IN THE GANGLION CELL LAYER IN RETINITIS PIGMENTOSA. Retina. 2023;43(8):1348–55. https://doi.org/10.1097/iae.0000000000003801.
Jingjing L, Jiao L, Xiang Z, Peiquan Z. Lamellar hole-associated epiretinal membrane is a common feature of macular holes in retinitis pigmentosa. Eye (London, England). 2020;34(4):643–9.
Makiyama Y, Oishi A, Otani A, Ogino K, Nakagawa S, Kurimoto M, Yoshimura N. Prevalence and spatial distribution of cystoid spaces in retinitis pigmentosa: investigation with spectral domain optical coherence tomography. Retina. 2014;34(5):981–8. https://doi.org/10.1097/iae.0000000000000010.
Gaudric A, Audo I, Vignal C, Couturier A, Boulanger-Scemama É, Tadayoni R, Cohen SY. Non-vasogenic cystoid maculopathies. Prog Retin Eye Res. 2022;91:101092. https://doi.org/10.1016/j.preteyeres.2022.101092.
Strong S, Liew G, Michaelides M. Retinitis pigmentosa-associated cystoid macular oedema: pathogenesis and avenues of intervention. Br J Ophthalmol. 2017;101(1):31–7. https://doi.org/10.1136/bjophthalmol-2016-309376.
Xu X, Fang Y, Yokoi T, Shinohara K, Hirakata A, Iwata T, Tsunoda K, Jonas JB, Ohno-Matsui K. Posterior staphylomas in eyes with retinitis pigmentosa without high myopia. Retina. 2019;39(7):1299–304. https://doi.org/10.1097/iae.0000000000002180.
Ruiz-Medrano J, Montero JA, Flores-Moreno I, Arias L, García-Layana A, Ruiz-Moreno JM. Myopic maculopathy: Current status and proposal for a new classification and grading system (ATN). Prog Retin Eye Res. 2019;69:80–115. https://doi.org/10.1016/j.preteyeres.2018.10.005.
Cheong KX, Xu L, Ohno-Matsui K, Sabanayagam C, Saw SM, Hoang QV. An evidence-based review of the epidemiology of myopic traction maculopathy. Surv Ophthalmol. 2022;67(6):1603–30. https://doi.org/10.1016/j.survophthal.2022.03.007.
Eun JS, Choi YJ, Kang SW, Choi KJ, Kim SJ, Roh HC. EN-FACE IMAGING OF ATYPICAL EPIRETINAL TISSUE IN LAMELLAR MACULAR HOLE. Retina. 2022;42(2):298–305. https://doi.org/10.1097/iae.0000000000003303.
Pang CE, Maberley DA, Freund KB, White VA, Rasmussen S, To E, Matsubara JA. LAMELLAR HOLE-ASSOCIATED EPIRETINAL PROLIFERATION: A Clinicopathologic Correlation. Retina. 2016;36(7):1408–12. https://doi.org/10.1097/iae.0000000000001069.
Yang YS, Lee JS, Son G, Sohn J. Epiretinal Proliferation Associated with Lamellar Hole or Macular Hole: Origin and Surgical Prognosis. Korean J Ophthalmol. 2019;33(2):142–9. https://doi.org/10.3341/kjo.2018.0070.
Pang CE, Spaide RF, Freund KB. Comparing functional and morphologic characteristics of lamellar macular holes with and without lamellar hole-associated epiretinal proliferation. Retina. 2015;35(4):720–6. https://doi.org/10.1097/iae.0000000000000390.
Asaad SZ. Full-Thickness Macular Hole Progressing from Lamellar Macular Hole with Epiretinal Proliferation. Case Rep Ophthalmol. 2021;12(1):134–41. https://doi.org/10.1159/000514526.
Yang JM, Choi SU, Kim YJ, Kim R, Yon DK, Lee SW, Shin JI, Lee JY, Kim JG. ASSOCIATION BETWEEN EPIRETINAL MEMBRANE, EPIRETINAL PROLIFERATION, AND PROGNOSIS OF FULL-THICKNESS MACULAR HOLE CLOSURE. Retina. 2022;42(1):46–54. https://doi.org/10.1097/iae.0000000000003262.
Da Mata AP, Burk SE, Foster RE, Riemann CD, Petersen MR, Nehemy M, Augsburger JJ. Long-term follow-up of indocyanine green-assisted peeling of the retinal internal limiting membrane during vitrectomy surgery for idiopathic macular hole repair. Ophthalmology. 2004;111(12):2246–53. https://doi.org/10.1016/j.ophtha.2004.05.037.
Anshu A, Apoorv G, Akhilesh A. Macular buckling and pars plana vitrectomy for retinitis pigmentosa with posterior staphyloma and macular hole. Indian J Ophthalmol Case Rep. 2021;1(4):694–6.
Panagiotou ES, Papathomas T, Nikopoulos K, Koukoula S, Quinodoz M, Rehman AU, Giannopoulos T, Rivolta C, Konstas AG. Management of Full-Thickness Macular Hole in A Genetically Confirmed Case with Usher Syndrome. Ophthalmol Ther. 2020;9(3):677–84. https://doi.org/10.1007/s40123-020-00276-4.
Miura G, Baba T, Yamamoto S. Two cases with retinitis pigmentosa that developed severe retinal atrophy long after vitreo-retinal surgery. Am J Ophthalmol Case Rep. 2020;18: 100716. https://doi.org/10.1016/j.ajoc.2020.100716.
Seungmo K, Hyung YJ, Gone KJ. Surgical repair of a full-thickness macular hole in retinitis pigmentosa: a case report. J Korean Ophthalmol Soc. 2019; 60(3):287.
Enani L, Kozak I, Abdelkader E. A Case of Unilateral Retinitis Pigmentosa Associated with Full Thickness Macular Hole. Middle East Afr J Ophthalmol. 2017;24(2):113–5. https://doi.org/10.4103/meajo.MEAJO_97_17.
Rush RB, Rush SW. BILATERAL LAMELLAR MACULAR HOLE SURGERY IN RETINITIS PIGMENTOSA. Retin Cases Brief Rep. 2016;10(1):83–5. https://doi.org/10.1097/icb.0000000000000166.
Vingolo EM, Valente S, Gerace E, Spadea L, Nebbioso M. Macular hole in retinitis pigmentosa patients: microincision vitrectomy with polydimethylsiloxane as possible treatment. Eye (Lond). 2015;29(5):699–702. https://doi.org/10.1038/eye.2014.344.
Ratra D, Raval V. Surgery for macular holes associated with unusual concomitant pathologies. Oman J Ophthalmol. 2013;6(2):112–5. https://doi.org/10.4103/0974-620x.116648.
Miriam GF, Joaquín CN, Antonio BF. Unilateral recurrent macular hole in a patient with retinitis pigmentosa: a case report. J Med Case Rep. 2013;7(1):69.
Hagiwara A, Yamamoto S, Ogata K, Sugawara T, Hiramatsu A, Shibata M, Mitamura Y. Macular abnormalities in patients with retinitis pigmentosa: prevalence on OCT examination and outcomes of vitreoretinal surgery. Acta Ophthalmol. 2011;89(2):e122-125. https://doi.org/10.1111/j.1755-3768.2010.01866.x.
Jin ZB, Gan DK, Xu GZ, Nao IN. Macular hole formation in patients with retinitis pigmentosa and prognosis of pars plana vitrectomy. Retina. 2008;28(4):610–4. https://doi.org/10.1097/IAE.0b013e31815ec341.
Nakamura H, Hayakawa K, Gaja T, Nagamine N, Sawaguchi K, Sawaguchi S. Vitreous surgery for macular hole in an eye with retinitis pigmentosa. Rinsho Ganka. 2005;59(8):1367–9.
Amemiya K, Takahashi M, Nishida A, Matsumura M, Hayakawa M, Honda Y. A macular hole in the eye of a young patient with retinitis pigmentosa. Nippon Ganka Gakkai Zasshi. 2002;106(4):236–42.
Saperstein DA. Sector retinitis pigmentosa with bitemporal visual field defects and macular hole. Retina. 2001;21(1):73–4. https://doi.org/10.1097/00006982-200102000-00016.
Ohashi H, Yamakawa R, Ohashi H. Retinal detachment associated with macular hole in a patient with high myopic Usher’s syndrome. Eye (Lond). 2001;15(Pt 3):335–6. https://doi.org/10.1038/eye.2001.109.
Yan F, Xia FJ, Jiang F, Yu HG. Visual and morphological outcomes of vitreomacular traction syndrome in retinitis pigmentosa treated by vitrectomy. Int J Ophthalmol. 2018;11(8):1411–5. https://doi.org/10.18240/ijo.2018.08.25.
Romano MR, Rossi T, Borgia A, Catania F, Sorrentino T, Ferrara M. Management of refractory and recurrent macular holes: A comprehensive review. Surv Ophthalmol. 2022;67(4):908–31. https://doi.org/10.1016/j.survophthal.2022.01.006.
Sippy BD, Engelbrecht NE, Hubbard GB, Moriarty SE, Jiang S, Aaberg TM Jr, Aaberg TM Sr, Grossniklaus HE, Sternberg P Jr. Indocyanine green effect on cultured human retinal pigment epithelial cells: implication for macular hole surgery. Am J Ophthalmol. 2001;132(3):433–5. https://doi.org/10.1016/s0002-9394(01)01061-3.
Sieving PA, Fishman GA. Refractive errors of retinitis pigmentosa patients. Br J Ophthalmol. 1978;62(3):163–7. https://doi.org/10.1136/bjo.62.3.163.
Lee SH, Yu HG, Seo JM, Moon SW, Moon JW, Kim SJ, Chung H. Hereditary and clinical features of retinitis pigmentosa in Koreans. J Korean Med Sci. 2010;25(6):918–23. https://doi.org/10.3346/jkms.2010.25.6.918.
Komori S, Ueno S, Ito Y, Sayo A, Meinert M, Kominami T, Inooka D, Kitagawa M, Nishida K, Takahashi K, et al. Steeper Macular Curvature in Eyes With Non-Highly Myopic Retinitis Pigmentosa. Invest Ophthalmol Vis Sci. 2019;60(8):3135–41. https://doi.org/10.1167/iovs.19-27334.
Kurata K, Hosono K, Hayashi T, Mizobuchi K, Katagiri S, Miyamichi D, Nishina S, Sato M, Azuma N, Nakano T et al: X-linked retinitis pigmentosa in Japan: clinical and genetic findings in male patients and female carriers. Int J Mol Sci. 2019;20(6). https://doi.org/10.3390/ijms20061518.
Akiba J, Konno S, Yoshida A. Retinal detachment associated with a macular hole in severely myopic eyes. Am J Ophthalmol. 1999;128(5):654–5. https://doi.org/10.1016/s0002-9394(99)00240-8.
Liu HY, Zou HD, Liu K, Song ZY, Xu X, Sun XD. Posterior vitreous cortex contributes to macular hole in highly myopic eyes with retinal detachment. Chin Med J (Engl). 2011;124(16):2474–9.
Ma J, Li H, Ding X, Tanumiharjo S, Lu L. Effectiveness of combined macular buckle under direct vision and vitrectomy with ILM peeling in refractory macular hole retinal detachment with extreme high axial myopia: a 24-month comparative study. Br J Ophthalmol. 2017;101(10):1386–94. https://doi.org/10.1136/bjophthalmol-2016-310123.
Theodossiadis GP, Sasoh M. Macular buckling for retinal detachment due to macular hole in highly myopic eyes with posterior staphyloma. Retina. 2002;22(1):129. https://doi.org/10.1097/00006982-200202000-00030.
Raja M, Goldsmith C, Burton BJ. Spontaneous resolution of full-thickness macular hole in retinitis pigmentosa. BMJ Case Rep. 2011;2011. https://doi.org/10.1136/bcr.03.2011.3999.
Acknowledgements
Not applicable.
Funding
No funding was received from any person, institute or organization to conduct the study.
Author information
Authors and Affiliations
Contributions
SYL and SYY concept and design, made the interpretation of the information, and drafted the initial manuscript. WSH and XMH carried out the collection of the reported studies. ZYT , ZJ and CY reviewed and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This article has been reviewed and approved by the Medical Ethics Committee of Qilu Hospital of Shandong University. It adheres to the tenets of the declaration of Helsinki. Written informed consent has been provided by all patient and confidentiality of the patients identity is maintained as much as possible.
Consent for publication
Written informed consent was obtained from the patients in this case series for publication of this article and any accompanying images. A copy of the written consent is available for review by the Editor of this journal.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Song, Y., Zhang, Y., Si, Y. et al. Pre- and postoperative OCT features and surgical outcomes of advanced retinitis pigmentosa with macular hole: case series and literature review. BMC Ophthalmol 24, 370 (2024). https://doi.org/10.1186/s12886-024-03643-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12886-024-03643-y