Abstract
Background
The occurrence and progression of asthma can be influenced by the components in food. Our study aims to determine whether dietary antioxidant and inflammatory potential are associated with the risk of mortality in asthma patients.
Methods
Participants from the 2001–2018 National Health and Nutrition Examination Survey (NHANES) aged 20 years and older with a diagnosis of asthma were included. Mortality status was obtained according to death certificate records from the National Death Index. The antioxidant and inflammatory potential of the diet was assessed using two widely used and dependable indices, Composite Dietary Antioxidant Index (CDAI) and Dietary Inflammatory Index (DII). Restricted cubic spline (RCS) regression was used to analyze the non-linear relationship between the two indexes and mortality. Multivariable Cox proportional risk models were used to estimate hazard ratio and 95% confidence intervals for mortality. Finally, the relationship between CDAI and DII was analyzed.
Results
A total of 4698 NHANES participants represented 23.2 million non-institutionalized residents of the US were enrolled in our study. Patients with higher CDAI or lower DII exhibited longer survival times. RCS regression showed a linear relationship of CDAI or DII with mortality. In the Cox regression, both crude and adjusted models demonstrated that higher CDAI or lower DII was linked to a reduced risk of all-cause mortality. Similar associations were found in subgroup analysis. Finally, a negative relationship was found between CDAI and DII.
Conclusion
Reducing pro-inflammatory or increasing antioxidant diets could reduce all-cause mortality among adult asthma patients.
Similar content being viewed by others
Introduction
Asthma is a common condition characterized by chronic inflammation of the lower respiratory tract, leading to recurrent episodes of wheezing, dyspnea, chest tightness, and coughing [1]. This condition affects individuals of all ages, with varying severity, and can lead to severe morbidity and mortality if not managed effectively [2]. The pathogenesis of asthma involves a complex interplay between genetic predisposition and environmental factors such as allergens, pollutants, respiratory infections, and occupational exposures. It has been shown that adults with asthma face a higher risk of mortality from chronic conditions compared to individuals without asthma, and asthma remains a significant cause of mortality [3, 4]. In light of the significant health risks associated with asthma, exploring potential strategies to mitigate mortality rates is imperative for improving patient outcomes and reducing the global burden.
The antioxidants protect the lungs from a wide variety of oxidants/reactive oxygen species, and antioxidant imbalance can lead to a variety of airway diseases like asthma, chronic obstructive pulmonary disease (COPD), and idiopathic pulmonary fibrosis [5]. Concurrently, sustained inflammation contributes to the pathogenesis and progression of asthma, involving the activation of eosinophils and mast cells [6]. Current research indicates that improving the inflammatory and antioxidant status of the diet can reduce the risk of mortality in patients with various diseases [7,8,9]. However, similar efforts among patients with asthma have not yet been conducted.
In this study, our primary goal was to determine whether the antioxidant and inflammatory potential of diet was associated with the risk of mortality in patients with asthma via using available data of the National Health and Nutrition Examination Surveys (NHANES), a nationwide survey of US residents that employs standardized protocols for laboratory examinations and sophisticated analytic approaches.
The antioxidant and inflammatory potential of the diet was assessed using two widely used and dependable indices, Composite Dietary Antioxidant Index (CDAI) and Dietary Inflammatory Index (DII).
Methods
Study design and population
NHANES, conducted by the Centers for Disease Control and Prevention (CDC), assesses the health and nutrition status of the US population through interviews, physical exams, and lab tests. It gathers data on demographics, dietary intake, medical history, and biomarkers. The database is vital for monitoring health trends, identifying disease risk factors, and informing public health policies. All of the NHANES data is accessible to the public and can be downloaded freely through: https://www.cdc.gov/nchs/nhanes/index.htm. The NHANES was approved by the National Center for Health Statistics Research Ethics Review Board, and written informed consent was obtained from all participants.
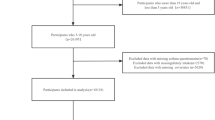
Data of NHANES 2001–2018 cycle were selected for analysis. Determination of asthma was based on Medical Conditions questionnaire. Participants answered the question of “Ever been told you have asthma”. Answering “yes” would be judged as having a previous history of asthma, and answering “no” would be judged as having no previous history of asthma. All participants with asthma were selected. Exclusion criteria included: individuals under the age of 20 years old; individuals with missing mortality data; individuals with missing dietary data or other covariates. Data of 4698 participants were ultimately included in this analysis (Fig. 1).
Calculation of CDAI and DII
24-hour dietary recall interviews were conducted to gather data on the intake of dietary antioxidants and other food components. Participants were asked to provide detailed dietary intake information for two 24-hour periods, which were then used to estimate intakes of energy, nutrients, and other food components. The first dietary recall was collected in an initial in-person interview during the NHANES visit, while the second was conducted via telephone 3 to 10 days later. For these analyses, the total estimated dietary intake of various nutrients was averaged over the two recall periods. If data for only the first day were available, that value was used instead of an average.
To assess the overall exposure to dietary antioxidants, a modified version of the CDAI, developed by Wright et al., was utilized, where a higher CDAI score indicates a higher total antioxidant capacity of the diet [10, 11]. This index was computed by aggregating six standardized dietary antioxidant intakes (vitamin A, vitamin C, vitamin E, zinc, selenium, and carotenoids), which were normalized by calculating the difference between individual intake and the mean, divided by the standard deviation as follows:
DII is a widely used score for assessing dietary inflammation by evaluating the inflammatory effects of 45 nutrients [12]. In this study, 28 of the 45 food parameters available in NHANES were used to calculate DII. Briefly, the calculation steps involved calculating the Z-score for each nutrient relative to global reference data [12]. Then the Z-score was converted to a distribution centered around zero and multiplied by the total inflammation score for each dietary component. Finally, the individual score for all nutrients were summed to obtain a comprehensive DII score, reflecting the overall dietary impact on inflammation, with lower scores indicating greater anti-inflammatory effects and higher scores implying stronger pro-inflammatory effects of the diet.
It should be noted that the CDAI and DII did not include those from supplements, medications, or plain drinking water. Additionally, the dietary interviews were conducted after the patients were aware of their asthma diagnosis.
Covariates
Covariates about individual characteristics included age, sex, race, body mass index (BMI), education level, smoke history, ratio of family income to poverty (PIR), alcohol intake, high blood pressure, diabetes, stroke history, coronary heart disease, cancer, and healthy eating index (HEI)-2015. All detailed measurement procedures are available at NHANES official website.
Participants who smoked at least 100 cigarettes in life were defined as smokers, regardless of whether he/she had quitted smoking at the time of interview. A BMI score less than 18.5 was categorized as underweight, 18.5 to 25 as normal weight, 25 to 30 as overweight, and above 30 as obesity. Participants who drink any type of alcoholic beverage less than 1 day per month in the past 12 months were considered non-drinkers.
Ascertainment of deaths
Data on all-cause mortality status were determined by using a probabilistic match between NHANES and the National Death Index (NDI) death certificate records. The follow-up time was defined as the period from being diagnosed with asthma to the date of death or to the end of follow-up (December 31, 2019).
Statistical analysis
Analysis was performed using appropriate NHANES sampling weights, and complex multistage cluster survey designs were taken into account. The baseline information was presented as frequency and percentage for categorical variables. Kaplan-Meier method was used to plot the survival curves associated with different classification methods for CDAI and DII. The log-rank test was used to compare differences between survival curves. Restricted cubic spline (RCS) regression with 4 knots (5th, 35th, 65th and 95th percentiles) was used to analyze the non-linear relationship between CDAI and mortality, as well as between DII and mortality.
Then, CDAI and DII were generally converted into categorical variables according to quartiles. Univariate and multivariable weighted Cox regression analyses were used to evaluate the hazard ratios (HRs) and 95% confidence intervals (CIs) for the association of CDAI and DII with all-cause mortality among participants with asthma. Meanwhile, we performed tests for linear trend in Cox regression by entering the median value of each category of CDAI and DII as a continuous variable in the models.
Subgroup analysis stratified by all covariates was conducted to explore whether the potential impact of CDAI or DII on mortality in the whole cohort was homogeneous across subgroups.
Spearman’s correlation analysis was used to calculate the correlation coefficients among CDAI, DII, PIR, BMI, HEI and age. Locally Weighted Scatterplot Smoothing (LOWESS) fit was used to fit the scatter plot data of CDAI and DII.
All multivariate models were adjusted for age, sex, race, BMI, education level, smoke history, PIR, alcohol intake, high blood pressure, diabetes, stroke history, coronary heart disease, cancer, and HEI. All statistical analyses were completed by R Project for Statistical Computing (version 4.3.3). A two-sided P value < 0.05 was defined as statistical significance.
Results
In total, 4698 NHANES participants represented 23.2 million non-institutionalized residents of the US. Of all citizens represented, women accounted for 59.2% (Table 1).
Kaplan-Meier survival curves were plotted after categorizing participants into two, three, and four groups based on variations in the CDAI or DII values. It was consistently observed that the groups with lower DII or higher CDAI exhibited better survival outcomes (P < 0.001, Fig. 2).
Survival curves using the Kaplan–Meier method for patients categorized by different classification methods. (a) half division of CDAI, (b) tertile division of CDAI, (c) quartile division of CDAI, (d) half division of DII, (e) tertile division of DII, (f) quartile division of DII. CDAI, composite dietary antioxidant index; DII, Dietary Inflammatory Index
To assess whether there was a linear relationship of CDAI or DII with all-cause mortality restricted cubic spline (RCS) analysis was conducted. The two indicators exhibited linear associations with all-cause mortality no matter in the crude model or the adjusted model (P for nonlinear > 0.05, Fig. 3).
Cox regression using restricted cubic spline regression among participants with asthma. (a) crude Cox regression between CDAI and all-cause mortality, (b) multivariable Cox regression between CDAI and all-cause mortality (c) crude Cox regression between DII and all-cause mortality, (d) multivariable Cox regression between DII and all-cause mortality. multivariable regression was adjusted by age, sex, race, body mass index, education level, smoke history, ratio of family income to poverty, alcohol intake, diabetes, stroke history, coronary heart disease, cancer, and healthy eating index. CI, confidence interval; CDAI, composite dietary antioxidant index; DII, Dietary Inflammatory Index
After stratifying CDAI and DII into quartiles and conducting weighted Cox regression analysis, both crude and adjusted models demonstrated that higher CDAI or lower DII was linked to a reduced risk of all-cause mortality. When comparing with the lowest CDAI quartile, the weighted multivariate hazard ratios (HRs) for all-cause mortality were, 0.88 (0.64–1.21) for Q2, 0.73 (0.52–1.01) for Q3, and 0.58 (0.41–0.81) for Q4 (P for trend = 0.001). When comparing with the lowest DII quartile, the weighted multivariate HRs for all-cause mortality were 1.46 (1.04–2.04) for Q2, 2.18 (1.57–3.04) for Q3, and 1.76 (1.22–2.54) for Q4 (P for trend < 0.001) (Table 2).
Weighted multivariable Cox regression was adjusted for age, sex, race, body mass index, education level, smoke history, ratio of family income to poverty, alcohol intake, high blood pressure, diabetes, stroke history, coronary heart disease, cancer, and healthy eating index. HR, hazard ratio; CI, confidence interval; CDAI, composite dietary antioxidant index; DII, Dietary Inflammatory Index.
Subgroup weighted Cox analysis stratified by age, sex, race, BMI, education level, smoke history, PIR, alcohol intake, high blood pressure, diabetes, stroke history, coronary heart disease, cancer, and dietary quality assessed by HEI was carried out in this study to further investigate the relationship between CDAI and DII with all-cause mortality among different populations with asthma. The results of subgroup analysis continued to indicate a consistent impact of CDAI and DII on all-cause mortality, similar to the findings in the overall population. As shown in Fig. 4, a higher CDAI was still found with a lower mortality in subgroup analysis. A similar association between elevated DII and increased mortality. Furthermore, the impact of higher DII scores on mortality appeared to be more pronounced among individuals with PIR > 3 compared to those with PIR < 3 (P for interaction = 0.012).
Subgroup analyses of multivariable adjusted association of CDAI or DII with all-cause mortality (weighted). HR: hazard ratio; CI: confidence interval; CDAI, composite dietary antioxidant index; DII, Dietary Inflammatory Index; PIR, Ratio of family income to poverty; BMI, body mass index; HEI, healthy eating index
Considering the contrasting effects of CDAI and DII on the risk of mortality among asthma participants, we further investigated the relationship between CDAI and DII. The result of Spearman’s correlation analysis showed a negative correlation between CDAI and DII (r = -0.876). This negative correlation between the two dietary indices was also observed in the LOWESS fit curves (Fig. 5).
The correlation matrix and scatter plot of CDAI and DII. (a) Spearman’s correlation analysis, (b) Scatter plot with LOWESS fit. CDAI, composite dietary antioxidant index; DII, Dietary Inflammatory Index; PIR, Ratio of family income to poverty; BMI, body mass index; HEI, healthy eating index; LOWESS, Locally Weighted Scatterplot Smoothing
Discussion
Our study was the first to analyze the effects of both CDAI and DII in the diet of asthma patients on the risk of mortality. This study showed that lower CDAI or higher DII was associated with a significantly elevated risk of all-cause mortality in participants with asthma, and the associations were significant after adjusting for age, sex, race, BMI, education level, smoke history, PIR, alcohol intake, high blood pressure, diabetes, stroke history, coronary heart disease, cancer, and HEI. We further found that in different subgroups, this relationship still exists, which could promise the reliability of the study. Finally, a negative correlation was found between CDAI and DII in participants’ diets, which to some extent explains the contrasting effects of these two indices on the risk of mortality.
Asthma imposes a significant burden on both individuals and populations, with excess mortality linked to various factors [4, 13]. The immunopathology of asthma involves the activation of both innate and adaptive immune systems, leading to the sustained stimulation of chronic airway inflammation. Following chronic airway inflammation, airway edema, excessive mucus production, mucus blockage, and structural alterations in the airways occur [14]. The severity and control of asthma, alongside factors like age, diet and environment, also influence the mortality rate of asthma patients [4, 15,16,17,18]. Several randomized controlled trials (RCTs) have consistently demonstrated that adopting a healthy diet confers benefits for the treatment of adult asthma patients [19,20,21]. We further explored the effects of antioxidant and pro-inflammatory diets on the mortality rate of asthma patients.
Chronic inflammation is characterized by persistent activation of inflammatory responses and tissue damage. This process induces oxidative stress, which in turn reduces cellular antioxidant capacity and leads to an overproduction of free radicals, ultimately impairing cellular function [22]. Consequently, inflammation and antioxidant capacity are closely intertwined in pathophysiology. Furthermore, dietary components can influence both inflammation and antioxidant capacity in the body [23, 24].
Some studies suggest that reactive oxygen species (ROS) play a critical role in initiating and amplifying airway inflammation in asthma [25, 26]. The CDAI aims to comprehensively assess the antioxidant capacity of food intake in populations. Current research has indicated potential benefits of moderate dietary total antioxidant intake for early-stage chronic kidney disease patients [27]. Meta-analyses have also suggested that adhering to a diet high in antioxidant properties may reduce the risk of all-cause mortality [28]. However, whether the magnitude of dietary antioxidant capacity affects mortality rates in asthma patients has not been studied. This study is the first to discover that increasing the CDAI contributes to reducing all-cause mortality in asthma patients.
Inflammation plays a crucial role in the development of asthma, and research has demonstrated that dietary nutrients can impact systemic inflammation levels. The DII serves as an indicator of the inflammatory potential of nutrient intake [12]. In the NHANES database, 28 components can use to calculate DII. Higher DII has been demonstrated to be associated with the occurrence of various diseases, including coronary heart disease, hypertension, and stroke [29,30,31,32]. Some studies have established associations between DII and asthma occurrence, as well as associations with reduced muscle mass and lung function in asthma patients [33, 34]. A recent analysis of the NHANES database suggested an impact of DII on mortality rates among asthma patients [24]. However, the study had a relatively simple analysis and did not account for the complex sampling characteristics of the NHANES database. Our study further expanded the sample size and utilized more accurate estimation methods. Ultimately, it concluded that higher DII intake is associated with an increased risk of mortality among asthma patients.
The correlation analysis between CDAI and DII revealed a negative association, suggesting both a negative relationship between these factors in diet and potential reasons for the differential impact of DII and CDAI on asthma patients. Therefore, enhancing dietary antioxidants while reducing pro-inflammatory components may not be mutually exclusive in practical terms and could be relatively achievable. Because each food contains both pro-inflammatory and antioxidant components, and these two have a strong correlation, we did not conduct an analysis of their synergistic effects. In summary, this study analyzed the effects of CDAI and DII on the prognosis of asthma patients and investigated the relationship between these two distinct indices, filling a gap in current research.
The top food contributors to the Dietary Inflammatory Index (DII) are refined carbohydrates, red and processed meats, fried foods, sugary beverages, high-fat dairy products, and some other foods that are rich in carbohydrates and fats [35, 36]. Fruits and vegetables, low-fat and high-fiber diet, like the Mediterranean diet could reduce dietary-induced inflammation in the body [37]. Consuming a diet rich in vegetables, fruits, and legumes, which are high in fiber and vitamin, can be more effective in combating oxidative stress and related issues [38]. We recommend that asthma patients purposefully increase their intake of foods with antioxidant potential in their daily diet and reduce their consumption of pro-inflammatory foods.
This study also has several limitations. Firstly, the NHANES findings relied on self-reports from patients, potentially introducing recall bias. The dietary data only reflected short-term dietary habits, limiting our ability to analyze time-varying dietary changes related to mortality. Secondly, while the study results were adjusted for age, sex, PIR, smoke, and other confounding factors, there could still be unknown confounding factors influencing the analyses. Thirdly, due to reasons such as patient privacy protection, the NHANES database lacks mortality data among underage patients. Therefore, this study did not analyze this subset of participants. Fourth, given that dietary habits vary across different geographical regions and among specific population groups, this factor may influence the analysis of the correlation between CDAI and DII.
Conclusion
The research findings indicate that reducing pro-inflammatory or increasing antioxidant diets can reduce all-cause mortality among adult asthma patients. We hope that this study can provide some recommendations for improving the prognosis of asthma patients and offer insights for future clinical research.
Data availability
The publicly available data used in this study can be found here: https://www.cdc.gov/nchs/nhanes/index.htm.
References
Mims JW. Asthma: definitions and pathophysiology. Int Forum Allergy Rhinology. 2015;5(Suppl 1) :S2-6. https://doi.org/10.1002/alr.21609.
Caffrey Osvald E, Bower H, Lundholm C, Larsson H, Brew BK, Almqvist C. Asthma and all-cause mortality in children and young adults: a population-based study. Thorax. 2020;75:1040–6. https://doi.org/10.1136/thoraxjnl-2020-214655.
To T, Simatovic J, Zhu J, Feldman L, Dell SD, Lougheed MD, Licskai C, Gershon A. Asthma deaths in a large provincial health system. A 10-year population-based study. Annals Am Thorac Soc. 2014;11:1210–7. https://doi.org/10.1513/AnnalsATS.201404-138OC.
Sidebotham HJ, Roche WR. Asthma deaths; persistent and preventable mortality. Histopathology. 2003;43:105–17. https://doi.org/10.1046/j.1365-2559.2003.01664.x.
Rahman I, Biswas SK, Kode A. Oxidant and antioxidant balance in the airways and airway diseases. Eur J Pharmacol. 2006;533:222–39. https://doi.org/10.1016/j.ejphar.2005.12.087.
Miller RL, Grayson MH, Strothman K. Advances in asthma: New understandings of Asthma’s natural history, risk factors, underlying mechanisms, and clinical management. J Allergy Clin Immunol. 2021;148:1430–41. https://doi.org/10.1016/j.jaci.2021.10.001.
Zhang J, Feng Y, Yang X, Li Y, Wu Y, Yuan L, Li T, Hu H, Li X, Huang H, et al. Dose-response association of dietary inflammatory potential with all-cause and cause-specific mortality. Advances Nutr (Bethesda. Md). 2022;13:1834–45. https://doi.org/10.1093/advances/nmac049.
Sheng L-T, Jiang Y-W, an, Pan. Koh W-P. Dietary total antioxidant capacity and mortality outcomes: the Singapore Chinese health study. European J Nutr. 2022; 61:2375–82. https://doi.org/10.1007/s00394-022-02812-3
Wang L, Yi Z. Association of the Composite dietary antioxidant index with all-cause and cardiovascular mortality: a prospective cohort study. Front Cardiovasc Med. 2022;9:993930. https://doi.org/10.3389/fcvm.2022.993930.
Wright ME, Mayne ST, Stolzenberg-Solomon RZ, Li Z, Pietinen P, Taylor PR, Virtamo J, Albanes D. Development of a comprehensive dietary antioxidant index and application to lung cancer risk in a cohort of male smokers. Am J Epidemiol. 2004;160:68–76. https://doi.org/10.1093/aje/kwh173.
Maugeri A, Hruskova J, Jakubik J, Kunzova S, Sochor O, Barchitta M, Agodi A, Bauerova H, Medina-Inojosa JR, Vinciguerra M. Dietary antioxidant intake decreases carotid intima media thickness in women but not in men: a cross-sectional assessment in the Kardiovize study. Free Radic Biol Med. 2019;131:274–81. https://doi.org/10.1016/j.freeradbiomed.2018.12.018.
Shivappa N, Steck SE, Hurley TG, Hussey JR, Hébert JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014;17:1689–96. https://doi.org/10.1017/S1368980013002115.
Kuo C-J, Chen VC-H, Lee W-C, Chen WJ, Ferri CP, Stewart R, Lai T-J, Chen C-C, Wang T-N, Ko Y-C. Asthma and suicide mortality in young people: a 12-year follow-up study. Am J Psychiatry. 2010;167:1092–9. https://doi.org/10.1176/appi.ajp.2010.09101455.
Gans MD, Gavrilova T. Understanding the immunology of asthma: pathophysiology, biomarkers, and treatments for asthma endotypes. Paediatr Respir Rev. 2020;36:118–27. https://doi.org/10.1016/j.prrv.2019.08.002.
Crane J, Pearce N, Burgess C, Woodman K, Robson B, Beasley R. Markers of risk of asthma death or readmission in the 12 months following a hospital admission for asthma. Int J Epidemiol. 1992;21:737–44. https://doi.org/10.1093/ije/21.4.737.
Tupper OD, Ulrik CS. Long-term predictors of severe exacerbations and mortality in a cohort of well-characterised adults with asthma. Respir Res. 2021;22:269. https://doi.org/10.1186/s12931-021-01864-z.
Pelkonen MK, Notkola I-LK, Laatikainen TK, Jousilahti P. 30-year trends in asthma and the trends in relation to hospitalization and mortality. Respir Med. 2018;142:29–35. https://doi.org/10.1016/j.rmed.2018.07.012.
Julia V, Macia L, Dombrowicz D. The impact of diet on asthma and allergic diseases. Nat Rev Immunol. 2015;15:308–22. https://doi.org/10.1038/nri3830.
Wood LG, Garg ML, Smart JM, Scott HA, Barker D, Gibson PG. Manipulating antioxidant intake in asthma: a randomized controlled trial. Am J Clin Nutr. 2012;96:534–43. https://doi.org/10.3945/ajcn.111.032623.
Sexton P, Black P, Metcalf P, Wall CR, Ley S, Wu L, Sommerville F, Brodie S, Kolbe J. Influence of mediterranean diet on asthma symptoms, lung function, and systemic inflammation: a randomized controlled trial. J Asthma: Official J Association Care Asthma. 2013;50:75–81. https://doi.org/10.3109/02770903.2012.740120.
Ma J, Strub P, Lv N, Xiao L, Camargo CA, Buist AS, Lavori PW, Wilson SR, Nadeau KC, Rosas LG. Pilot randomised trial of a healthy eating behavioural intervention in uncontrolled asthma. Eur Respir J. 2016;47:122–32. https://doi.org/10.1183/13993003.00591-2015.
Pérez-Torres I, Guarner-Lans V, Rubio-Ruiz ME. Reductive stress in inflammation-associated diseases and the pro-oxidant effect of antioxidant agents. Int J Mol Sci. 2017;18. https://doi.org/10.3390/ijms18102098.
Chen R, Liu H, Zhang G, Zhang Q, Hua W, Zhang L, Lv N, Zhang Y, Dai D, Zhao R, et al. Antioxidants and the risk of stroke: results from NHANES and two-sample mendelian randomization study. Eur J Med Res. 2024;29:50. https://doi.org/10.1186/s40001-024-01646-5.
Yuan Y, Ye W, Fang X. Dietary inflammatory index and all-cause mortality among asthma patients: a population-based cohort study. Annals Palliat Med. 2022;11:2061–70. https://doi.org/10.21037/apm-22-615.
Michaeloudes C, Abubakar-Waziri H, Lakhdar R, Raby K, Dixey P, Adcock IM, Mumby S, Bhavsar PK, Chung KF. Molecular mechanisms of oxidative stress in asthma. Mol Aspects Med. 2022;85:101026. https://doi.org/10.1016/j.mam.2021.101026.
Nadeem A, Masood A, Siddiqui N. Oxidant—antioxidant imbalance in asthma: scientific evidence, epidemiological data and possible therapeutic options. Ther Adv Respir Dis. 2008;2:215–35. https://doi.org/10.1177/1753465808094971.
Li Y, Ling G-C, Ni R-B, Ni S-H, Sun S-N, Liu X, Deng J-P, Ou-Yang X-L, Li J, Xian S-X, et al. Association of dietary total antioxidant capacity with all-cause and cardiovascular mortality in patients with chronic kidney disease: based on two retrospective cohort studies of NHANES. Ren Fail. 2023;45:2205950. https://doi.org/10.1080/0886022X.2023.2205950.
Jayedi A, Rashidy-Pour A, Parohan M, Zargar MS, Shab-Bidar S. Dietary antioxidants, circulating antioxidant concentrations, total antioxidant capacity, and risk of all-cause mortality: a systematic review and dose-response Meta-analysis of prospective observational studies. Advances in nutrition (Bethesda. Md). 2018;9:701–16. https://doi.org/10.1093/advances/nmy040.
Zhang J, Jia J, Lai R, Wang X, Chen X, Tian W, Liu Q, Li J, Ju J, Xu H. Association between dietary inflammatory index and atherosclerosis cardiovascular disease in U.S. adults. Front Nutr. 2022;9(1044329). https://doi.org/10.3389/fnut.2022.1044329.
Mao Y, Weng J, Xie Q, Wu L, Xuan Y, Zhang J, Han J. Association between dietary inflammatory index and stroke in the US population: evidence from NHANES 1999–2018. BMC Public Health. 2024;24:50. https://doi.org/10.1186/s12889-023-17556-w.
Wu L, Shi Y, Kong C, Zhang J, Chen S. Dietary inflammatory index and its association with the prevalence of coronary heart disease among 45,306 US adults. Nutrients. 2022;14. https://doi.org/10.3390/nu14214553.
Zhou N, Xie Z-P, Liu Q, Xu Y, Dai S-C, Lu J, Weng J-Y, Wu L-D. The dietary inflammatory index and its association with the prevalence of hypertension: a cross-sectional study. Front Immunol. 2022;13:1097228. https://doi.org/10.3389/fimmu.2022.1097228.
Wood LG, Shivappa N, Berthon BS, Gibson PG, Hebert JR. Dietary inflammatory index is related to asthma risk, lung function and systemic inflammation in asthma. Clin Experimental Allergy J Br Soc Allergy Clin Immunol. 2015;45:177–83. https://doi.org/10.1111/cea.12323.
Lin S, Su X, Chen L, Cai Z. Association of dietary inflammatory index with Sarcopenia in asthmatic patients: a cross-sectional study. Front Nutr. 2023;10:1215688. https://doi.org/10.3389/fnut.2023.1215688.
Stromsnes K, Correas AG, Lehmann J, Gambini J, Olaso-Gonzalez G. Anti-inflammatory properties of Diet: role in healthy aging. Biomedicines 2021; 9. https://doi.org/10.3390/biomedicines9080922.
Fritsch J, Garces L, Quintero MA, Pignac-Kobinger J, Santander AM, Fernández I, Ban YJ, Kwon D, Phillips MC, Knight K, et al. Low-Fat, High-Fiber Diet reduces markers of inflammation and dysbiosis and improves quality of life in patients with Ulcerative Colitis. Clinical gastroenterology and hepatology: the official clinical practice. J Am Gastroenterological Association. 2021;19:1189–e119930. https://doi.org/10.1016/j.cgh.2020.05.026.
Galland L. Diet and inflammation. Nutr Clin Practice: Official Publication Am Soc Parenter Enter Nutr. 2010;25:634–40. https://doi.org/10.1177/0884533610385703.
Chen X, Li H, Zhang B, Deng Z. The synergistic and antagonistic antioxidant interactions of dietary phytochemical combinations. Crit Rev Food Sci Nutr. 2022;62:5658–77. https://doi.org/10.1080/10408398.2021.1888693.
Acknowledgements
We would like to thank the staff of the National Health and Nutrition Examination Surveys (NHANES) database and Centers for Disease Control and Prevention (CDC).
Funding
None.
Author information
Authors and Affiliations
Contributions
YQ G and LN H conceived and designed the study. YQ G and HX Z performed data analysis and interpretation. HX Z drafted the manuscript, which was critically revised by YQ G and LN H.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study based on NHANES was approved by the National Center for Health Statistics Research Ethics Review Board and was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Conflict of interest
None.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, H., Huang, L. & Guo, Y. Dietary antioxidant and inflammatory potential in asthmatic patients and its association with all-cause mortality. Nutr J 23, 95 (2024). https://doi.org/10.1186/s12937-024-00994-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12937-024-00994-6