Abstract
Background
Activation of the IL-33/ST2 axis leads to the production of proinflammatory cytokines and thus to the triggering of osteoclastogenesis, which is why it plays an important role in the immunopathogenesis of periodontitis. The aim of this study was to compare IL-33 levels in serum, plasma, saliva and gingival crevicular fluid (GCF) of subjects with chronic periodontitis (CP) in comparison with the control group (CG).
Methods
This systematic review and meta-analysis followed the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) and was registered in the Open Science Framework (OSF): https://doi.org/10.17605/OSF.IO/YHUWA. Six electronic databases were used for study identification; PubMed, Google Scholar, ScienceDirect, Web of Science, Scopus and Dentistry & Oral Sciences Source from March 10, 2012 to April 30, 2024. The Joanna Briggs Institute (JBI) tool was used to assess the quality of the included cross-sectional articles and clinical trials.
Results
Of the 949 articles identified, 14 were included according to the inclusion and exclusion criteria. The total number of individuals studied in the included investigations was 814 of whom 445 had CP and 369 were healthy. The reported age range was from 20 to 50 years, with a mean age ± standard deviation of 40.29 ± 7.83 years. Four hundred and twenty-six (52%) patients were men and 388 (48%) were women. Meta-analysis revealed that there is an increase in IL-33 levels in plasma, saliva and GCF of subjects with CP compared to CG (p = * < 0.05).
Conclusions
This study found a significant increase in IL-33 levels in different biological samples (plasma, saliva and GCF) of individuals with CP compared to CG, thus IL-33 has potential to be a biomarker in the diagnosis of periodontitis.
Similar content being viewed by others
Background
Periodontitis is an oral infection characterized by the destruction of the soft and hard tissues that support the teeth [1]. This disease is associated with the presence of a polymicrobial dysbiotic film constituted by a high prevalence of periodontopathogens (Aggregatibacter actinomycetemcomitans, Fusobacterium nucleatum, Tannerella forsythia, Porphyromonas gingivalis and Treponema denticola) and regulated in turn by immunoinflammatory factors [2]. Its high incidence worldwide is worrying and alarming because it is one of the leading causes of tooth loss, making it a major public health problem that remains unresolved [3]. Both the diagnosis and treatment of periodontitis are well established, however, there are still gaps in knowledge about the molecular mechanisms involved in the regulation of the inflammatory and destructive process of the periodontium, therefore, knowing these aspects will greatly facilitate scientists and clinicians in precision treatment [4].
Interleukin 33 (IL-33) or alarmin is a proinflammatory cytokine, a member of the IL-1 family, which plays an important role in human inflammation and in particular in the periodontal immune response [5]. IL-33 is encoded by the IL33 gene located on 9p24.1 [6]. Regarding the gene structure, it has its promoter, eight exons, two introns and its 5'UTR and 3'UTR regions with a total of 4,200 bp [7]. IL33 mRNA encodes a protein of 270 amino acids (aa) with a molecular weight of 30.75 kDa. Thus IL-33 consists of a nuclear domain (1-65aa), a core domain (66-112aa) and an IL-1-like domain (113-270aa) [8]. When released, IL-33 interacts and binds with the suppression of tumorigenicity 2 receptor (ST2) which is a member of the toll-like receptor (TLR)/IL-1 superfamily [9]. ST2 can present as a membrane-bound receptor (ST2L) and form a heterodimer complex together with the IL-1 receptor accessory protein (IL-1RAcP co-receptor) or it can present in its soluble form (sST2) and act as a decoy receptor by binding to IL-33 to antagonize the effects of ST2L [10]. Mast cells, fibroblasts, dendritic cells, T cells and endothelial cells express ST2L on their membrane [11]. It is important to note that IL-33 has two main functions, on the one hand, in the nucleus, it can bind to chromatin and act as a nuclear factor, and on the other hand, when released (depending on the conditions), it can act as an anti- or pro-inflammatory mediator [12]. In the latter case, it has been shown that the IL-33/ST2 axis activates the nuclear factor kappa B (NF-κB) pathway contributing to the production of proinflammatory cytokines such as interleukin 1 (IL-1), tumor necrosis factor alpha (TNF-α), interleukin 1 beta (IL-1β) and interleukin 8 (IL-8) that participate in chemotaxis and regulate the process of osteoclastogenesis, two important events in periodontal inflammation and destruction [13].
IL-33 can be detected using various methodologies in biofluids such as serum, plasma, saliva and gingival crevicular fluid (GCF), thus it has diagnostic potential [14]. There are some publications showing that IL-33 concentrations vary in different biological samples from individuals with chronic periodontitis (CP) compared to the control group (CG) [14,15,16,17,18,19,20,21,22,23,24,25,26], however, there is still controversy whether IL-33 levels can be used as indicators to differentiate both conditions. Therefore, our working team undertook the task of constructing and presenting a meta-analysis that can contribute to the current knowledge on this topic.
Rationale objective
Based on clinical findings published in the literature, this systematic review and meta-analysis aimed:
To compare IL-33 levels in serum, plasma, saliva and GCF of individuals with CP and CG.
Materials and methods
Research type
Exploratory, descriptive and retrospective review.
Ethics
This systematic review and meta-analysis complies with ethical standards.
Registration
The protocol was registered in Open Science Framework (OSF) database, with registration number: https://doi.org/https://doi.org/10.17605/OSF.IO/YHUWA, accessed on June 26, 2024.
Protocol
The study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines [27].
PICOS and focus question
The PICOS items (Table 1) were used to formulate the following research question:
Is there an increase in IL-33 protein levels in serum, plasma, saliva and GCF samples from individuals with CP compared to CG?
Eligibility criteria
Inclusion criteria
This review included:
-
Original human studies with a cross-sectional design and clinical trials.
-
Studies that evaluated IL-33 protein levels in different biofluids such as serum, plasma, saliva and GCF of individuals with CP and CG.
-
Individuals in the exposure group (CP) had to have at least 15 teeth, as well as a bleeding on probing; % > 30%, probing deep; > 3 mm and clinical attachment level; > 2 mm.
-
Studies that used enzyme-linked immunosorbent assay (ELISA) and Multiplex bead immunoassays as the method of immunological analysis.
Exclusion criteria
This review excluded:
-
Any systemic condition that impaired the periodontal health of individuals.
-
Individuals undergoing orthodontic treatment.
-
Pregnant/breastfeeding or menopausal women.
-
Smokers, alcohol drinkers, as well as individuals on medication (antibiotics/immunosuppressors) within the last 3 months.
-
Studies in animal models and/or cell lines, mini-reviews, narrative, comprehensive or scoping reviews, meta-analyses, book chapters, letters to the editor, short communications, conference posters, thesis, dissertations and editorials, as they are not related to the objectives of this work.
Information sources and search strategy
The databases PubMed, Google Scholar, ScienceDirect, Web of Science, Scopus and Dentistry & Oral Sciences Source were consulted from March 10, 2012 to April 30, 2024. The search strategy employed for all databases is shown in Table 2. The following journals were consulted as part of the manual search: Journal of Periodontal Research, Journal of Periodontal and Implant Science e International Journal of Periodontics & Restorative Dentistry, Periodontology 2000, Journal of Clinical Periodontology y Journal of Periodontology.
Study selection
Article titles and abstracts were selected by two investigators (M.A.A.S and E.U.S.T) independently according to the application of the eligibility criteria. Relevant and potential articles for inclusion were retrieved for full-text evaluation. Finally, after discussion for any disagreement, consensus was reached and articles with relevant content were chosen.
Data collection
The variables included in this work were:
-
First and second author data.
-
Year of publication.
-
Study design.
-
Approval by the bioethics committee of the corresponding institution.
-
Name of the journal where the article was published.
-
Admissibility criteria.
-
Age.
-
Gender.
-
Number of cases with CP and CG.
-
Definition criteria for the group of individuals with CP.
-
Periodontal clinical parameters evaluated.
-
Type of biological sample.
-
Cytokine evaluated.
-
IL-33 values in individuals with CP and CG.
-
Main findings and conclusions.
All these data were captured by the same investigators mentioned above and in case of any disagreement, a third external investigator was consulted for resolution.
Synthesis methods
Quantitative variables were represented by mean and standard deviation, while qualitative variables were represented by absolute and relative frequency.
Assessment quality
Two investigators (M.A.A.S and A.H) assessed the quality of cross-sectional studies and clinical trials separately, using the Joanna Brigs Institute instrument [28]. Articles with a score of 1–3 were considered low quality, articles with a score of 4–6 were considered moderate quality, and articles with a score of 7–8 were considered high quality.
Statistical analysis
The STATA V.15 program (Stata Corp, College Station, TX, USA) was used to perform the data synthesis. For quantitative analysis, the standardized mean difference (SMD) of IL-33 protein levels (pg/ml) was calculated between both study groups (CP vs CG) and in four different biological samples (serum, plasma, saliva and GCF). A fixed effects model with a 95% confidence interval was used. Significance was adjusted to a value of *p < 0,05.
Results
Study selection
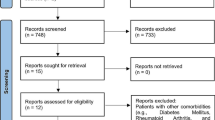
Initially 947 articles were found in the 6 electronic databases, including PubMed (n = 52), Google Scholar (n = 514), ScienceDirect (n = 355), Web of Science (n = 1), Scopus (n = 4) and Dentistry & Oral Science Source (n = 1). In the manual search, 2 more articles were found, giving a total of 949 articles. In the identification phase, duplicates were eliminated (n = 17). Next, based on title and abstract, the remaining 932 studies were reviewed. Applying the eligibility criteria, 319 more records were excluded (reviews n = 286; editorials n = 1; book chapters n = 29; short communications n = 3), giving a total of 613 potentially relevant records. After analyzing the full text of the remaining articles, 599 articles were excluded because they were outside the scope of the central topic. Therefore, a total of 14 articles were included for qualitative and quantitative analysis in the present review. Details of the study selection for this review are shown in Fig. 1.
Qualitative analysis
A total of 14 investigations [14,15,16,17,18,19,20,21,22,23,24,25,26] were reviewed in this study, of which 12 (86%) have a cross-sectional design [14,15,16, 19,20,21,22,23,24,25,26] and 2 (14%) are clinical trials [17, 18]. The total number of individuals studied in the included investigations was 814 of which 445 represented the case group (individuals with CP) and 369 represented the CG (periodontally healthy individuals). The ages of the individuals ranged from 20 to 50 years, with a mean age ± (SD) of 40.29 ± 7.83 years, of which 388 (48%) were female and 426 (52%) were male [14,15,16,17,18,19,20,21,22,23,24,25,26]. All investigations (100%) were approved by the corresponding bioethics committee [14,15,16,17,18,19,20,21,22,23,24,25,26]. The most frequent exclusion criteria was the presence of any systemic disease that could be influencing the periodontal condition of the individual [14,15,16,17,18,19,20,21,22,23,24,25,26]. Most of the articles were published after 2015 (11:79%) [17,18,19,20,21,22,23]. The oldest study was from 2012 [26], and the most recent from 2024 [17]. The 14 investigations were published in 8 different countries [14,15,16,17,18,19,20,21,22,23,24,25,26]. Four (29%) studies were conducted in Turkey [18, 22, 23, 25], three (21.5%) studies in India [17, 20], two (14.5%) studies in Iraq [14, 19], and the rest (7%) in China [15], Colombia [16] Egypt [21], USA [24] and Iran [26]. Table 3 also describes the names of the Journals where the articles were published.
The enzyme-linked immunosorbent assay (ELISA) was the most frequent immunological method employed by researchers for the determination of IL-33 protein levels (86%) [14, 15, 17,18,19,20,21,22,23, 25, 26], followed by the Multiplex bead (14%) [16, 24]. Most studies revealed that there is an increase in IL-33 concentrations in serum, plasma, saliva and GCF of individuals with chronic periodontitis compared to the healthy population [14, 17,18,19,20,21,22, 25, 26] (Table 4).
Quality
Eight (57%) studies presented moderate quality [20,21,22,23,24,25,26], while the rest (43%) presented high quality [14,15,16,17,18,19] according to JBI criteria (Figs. 2, 3).
Quantitative analysis of IL-33 levels in serum of individuals with chronic periodontitis vs control group
A meta-analysis of four studies [14, 15, 19, 26] was performed comparing IL-33 levels in serum of individuals with chronic periodontitis compared to healthy individuals: (SMD = 0.43 (95%CI = − 0.07–0.94); *p = 0.09). This means that serum IL-33 concentration in CP was higher than in CG, but without statistical significance. Study heterogeneity was low (I2 = 2.9%, *p = 0.78), so a fixed effects model was used (Fig. 4, panel A).
Quantitative analysis of IL-33 in plasma of individuals with chronic periodontitis vs control group
A meta-analysis of four studies [20, 22, 25] was performed comparing plasma IL-33 levels in individuals with chronic periodontitis compared to healthy individuals: (SMD = 4.45 (95%CI = 0.82–8.08); *p = 0.016). This means that plasma IL-33 concentration in CP was significantly higher than in CG. Study heterogeneity was low (I2 = 0.00%, *p = 0.493), so a fixed effects model was used (Fig. 4, panel B).
Quantitative analysis of IL-33 levels in saliva of individuals with chronic periodontitis vs control group
A meta-analysis of six studies [17,18,19, 21, 21, 22, 25] was performed comparing IL-33 levels in saliva of individuals with chronic periodontitis compared to healthy individuals: (SMD = 16.24 (95%CI = 4.09–28.40); *p = 0.009). This means that the concentration of IL-33 in saliva in CP was significantly higher than in CG. The heterogeneity of the studies was low (I2 = 4.7%, *p = 0.386), so a fixed effects model was used (Fig. 4, panel C).
Quantitative analysis of IL-33 levels in GCF of individuals with chronic periodontitis vs control group
A meta-analysis of eight studies [16, 19,20,21,22,23,24,25] was performed comparing IL-33 levels in GCF of individuals with chronic periodontitis compared to healthy individuals: (SMD = 19.30 (95%CI = 7.90–30.69); *p = 0.001). This means that the concentration of IL-33 in GCF in CPG was significantly higher than in CG. The heterogeneity of the studies was low (I2 = 44.3%, *p = 0.083), so a fixed effects model was used. The funnel plot and Egger’s test indicated that there was no publication bias (*p = 0.097) (Fig. 4, panel D and E).
Discussion
Periodontitis is characterized by inflammation and destruction of the teeth-supporting apparatus [29]. Initially, inflammation is driven by polymicrobial imbalance that triggers excessive activation of the host immune response [30]. Bacterial aggression and proinflammatory stimuli (extracellular ATP and mechanical stress) result in IL-33 production [31, 32]. As for bacterial challenge, pathogen-associated molecular patterns (PAMPs) present in the microenvironment of periodontal pockets of individuals with CP interact and bind to pattern recognition receptors (PRRs) inducing the expression of IL-33 and other proinflammatory mediators (TNF-α, IL-1, IL-6, IL-8, IL-17, CX3CL1) [33]. In fact, one study showed that fimbriae and lipopeptide from P. gingivalis are recognized by TLR-2 of dendritic cells leading to increased IL-33 production [34]. Another study found that gingipains also upregulate IL-33 expression in gingival epithelial cells through the PAR-2-PLC signaling pathway [35]. In this context, once IL-33 is released from the nucleus to the outside, it binds to its receptor ST2 which is expressed by different cell types (gingival fibroblasts, periodontal ligament cells, osteoblasts, osteoclasts) [36,37,38,39] allowing the recruitment of myeloid differentiation primary response protein (MyD88) which in turn recruits IL-1R-associated kinase 1 and 4 (IRAK-1/4) and tumor necrosis factor receptor-associated factor 6 (TRAF-6) [12]. This signaling cascade leads to the activation of PI3K, P38, AP-1, ERK and NF-κB, which ultimately promotes the expression of genes involved in the inflammatory process and bone metabolism in the periodontium [40].
Osteoclastic activity leads to bone loss compromising tooth health [41]. IL-33 has been shown to exacerbate periodontal disease through upregulation of receptor activator of nuclear factor kappa B ligand (RANKL). In this study, it was found that the percentages of RANKL expressing B and T cells in cervical draining lymph nodes were higher in P. gingivalis-infected mice treated with IL-33 compared to P. gingivalis-infected controls treated with phosphate-buffered saline, thus in the context of bacterial infection, IL-33 exacerbates bone loss in a RANKL-dependent manner [42].
Scientific evidence has shown that there are changes in IL-33 concentrations in different biological samples such as serum, plasma, saliva, gingival tissue and GCF [14,15,16,17,18,19,20,21,22,23,24,25,26]. In this systematic review, 12 cross-sectional studies and 2 clinical trials were included for the analysis of IL-33 in 4 biofluids (serum, plasma, saliva and GCF) corresponding to 445 individuals with CP and 369 healthy individuals. Regarding the serum samples, the meta-analysis revealed no statistically significant differences (p = > 0.05). Despite this, only two authors; Abdul-Wahab et al., 2024 [14] and Ladez et al., 2012 [26] demonstrated a remarkable elevation of serum IL-33 levels in individuals with CP compared to CG, as well as a positive correlation between serum levels of this cytokine with some clinical parameters such as plaque index, gingival index and bleeding on probing. In addition, another study found that subjects with severe periodontitis and poor oral hygiene were associated with elevated serum sST2 and C-reactive protein levels [43]. Contrary to these findings, Lin et al., 2024 [15] and Habeeb et al., 2021 [19] found that serum IL-33 levels were lower in subjects with CP compared to CG. Moreover, in the former study, a negative association was observed between serum levels of this cytokine with the risk of periodontitis in the Chinese population.
On the other hand, regarding plasma samples, the meta-analysis revealed that there are statistically significant differences between both study groups (p = * < 0.05). In this case, Ballambettu et al., 2019 [20], Pradeep and Pai et al., 2019 [20], Sağlam et al., 2015 [22] and Buduneli et al., 2013 [25] demonstrated that subjects with CP had high plasma IL-33 concentrations compared to periodontally healthy individuals. The high levels of IL-33 in serum and blood plasma could be a consequence of the increased expression of this cytokine in periodontal tissues, which enters the systemic circulation, since the individuals included in the studies had no systemic involvement [14, 15, 19, 20, 22, 22, 25, 26, 43].
In relation to saliva samples, the meta-analysis revealed that there are statistically significant differences between both study groups (p = * < 0.05). Renjith et al., 2023 [17], Yilmaz et al., 2023 [18], Habeeb et al., 2021 [19], Tarrat et al., 2018 [21] and Sağlam et al., 2015 [22] demonstrated that subjects with CP had high concentrations of IL-33 and sST2 in saliva compared to periodontally healthy individuals. In contrast to these findings, only one study [25] found elevated levels in healthy subjects and another study [16] did not detect IL-33 in saliva samples. The high levels of IL-33 in saliva could be a consequence of the increased expression of this cytokine in GCF, as a positive correlation between both biofluids has also been demonstrated.
In this review, a quantitative analysis of IL-33 protein levels in gingival tissue samples could not be performed due to the lack of studies. However, the literature has reported that in gingival tissue biopsies from subjects with CP there is an increased expression of IL-33 and its receptor ST2 compared to the healthy population [42].
Finally, regarding the GCF samples, the meta-analysis revealed that there are statistically significant differences between both study groups (p = * < 0.05). Habeeb et al., 2021 [19], Ballambettu et al., 2019 [20], Pradeep and Pai et al., 2019 [20], Tarrat et al., 2018 [21] and Sağlam et al., 2015 [22] demonstrated that subjects with CP had high concentrations of IL-33 in GCF compared to periodontally healthy individuals. In addition, another study found that subjects with peri-implantitis had elevated sST2 levels compared to CG [44]. Also, in subjects with periodontitis and diabetes mellitus, sST2 levels in GCF increased compared to subjects with only periodontitis and healthy controls [45]. In contrast to these findings, two studies [16, 25] found elevated levels of IL-33 in healthy subjects compared to subjects with CP and another study [24] did not detect IL-33 in GCF samples.
Limitations
The present systematic review and meta-analysis had some limitations as mentioned below:
-
The inclusion of a larger number of cross-sectional studies, as their level of evidence is lower than that of prospective studies. In addition, in this type of studies, temporal relationships are usually difficult to evaluate and statistically, they are studies that determine only the association between the variables of interest and causality cannot be determined.
-
The inclusion of a small number of individuals in clinical studies.
-
Differences in gender, age, genetic profile, metabolic, hormonal, microbiological, oral hygiene habits, as well as in the periodontal classification used as a reference to differentiate individuals with the disease (CP) from periodontally healthy individuals.
-
The use of two different methodologies (ELISA and Multiplex bead) for the analysis of IL-33 levels in serum, plasma, saliva and GCF samples.
-
Studies are needed to strengthen the methodological aspects to improve the quality of the results obtained.
Conclusions
The present systematic review and meta-analysis found a significant increase in IL-33 levels in different biological samples (plasma, saliva and GCF) from individuals with CP compared to CG, thus IL-33 has potential to be a biomarker in the diagnosis of periodontitis (Fig. 5).
Availability of data and materials
The data supporting this study’s findings are available from the corresponding author upon reasonable request.
Abbreviations
- IL-33:
-
Interleukin 33
- ST2:
-
Suppression of tumorigenicity 2 receptor
- TNF-α:
-
Tumor necrosis factor alpha
- IL-6:
-
Interleukin 6
- IL-1β:
-
Interleukin 1 beta
- IL-8:
-
Interleukin 8
- IL-17:
-
Interleukin 17
- CX3CL1:
-
Fractalkine
- GCF:
-
Gingival crevicular fluid
- RANKL:
-
Receptor activator of nuclear factor kappa B ligand
- PAMPs:
-
Pathogen-associated molecular patterns
- PRRs:
-
Pattern recognition receptors
- NF-κB:
-
Nuclear factor kappa B
- ELISA:
-
Enzyme-linked immunosorbent assay
- MyD88:
-
Myeloid differentiation primary response protein
- IRAK-1:
-
IL-1R-associated kinase 1
- IRAK-4:
-
IL-1R-associated kinase 4
- TRAF-6:
-
Tumor necrosis factor receptor-associated factor 6
- PI3K:
-
Phosphoinositide 3-kinase
- P38:
-
Mitogen-activated protein kinase
- AP-1:
-
Activating protein 1
- ERK:
-
Extracellular signal-regulated kinase
References
Ganimusa I, Chew E, Lu EM. Vitamin D deficiency, chronic kidney disease and periodontitis. Medicina. 2024;60(3):420. https://doi.org/10.3390/medicina60030420.
Di Stefano M, Polizzi A, Santonocito S, Romano A, Lombardi T, Isola G. Impact of oral microbiome in periodontal health and periodontitis: a critical review on prevention and treatment. Int J Mol Sci. 2022;23(9):5142. https://doi.org/10.3390/ijms23095142.
Fischer RG, Lira Junior R, Retamal-Valdes B, Figueiredo LC, Malheiros Z, Stewart B, Feres M. Periodontal disease and its impact on general health in Latin America. Section V: Treatment of periodontitis. Braz Oral Res. 2020;34(suppl 1):e026. https://doi.org/10.1590/1807-3107bor-2020.vol34.0026.
Bhuyan R, Bhuyan SK, Mohanty JN, Das S, Juliana N, Juliana IF. Periodontitis and its inflammatory changes linked to various systemic diseases: a review of its underlying mechanisms. Biomedicines. 2022;10(10):2659. https://doi.org/10.3390/biomedicines10102659.
Papathanasiou E, Conti P, Carinci F, Lauritano D, Theoharides TC. IL-1 Superfamily Members and Periodontal Diseases. J Dent Res. 2020;99(13):1425–34. https://doi.org/10.1177/0022034520945209.
IL33 - Interleukin-33 - Homo sapiens (Human) | UniProtKB | UniProt
Liew FY, Girard JP, Turnquist HR. Interleukin-33 in health and disease. Nat Rev Immunol. 2016;16(11):676–89. https://doi.org/10.1038/nri.2016.95.
Yi XM, Lian H, Li S. Signaling and functions of interleukin-33 in immune regulation and diseases. Cell Insight. 2022;1(4): 100042. https://doi.org/10.1016/j.cellin.2022.100042.
De la Fuente M, MacDonald TT, Hermoso MA. The IL-33/ST2 axis: role in health and disease. Cytokine Growth Factor Rev. 2015;26(6):615–23. https://doi.org/10.1016/j.cytogfr.2015.07.017.
Barksby HE, Lea SR, Preshaw PM, Taylor JJ. The expanding family of interleukin-1 cytokines and their role in destructive inflammatory disorders. Clin Exp Immunol. 2007;149(2):217–25. https://doi.org/10.1111/j.1365-2249.2007.03441.x.
Braun H, Afonina IS, Mueller C, Beyaert R. Dichotomous function of IL-33 in health and disease: from biology to clinical implications. Biochem Pharmacol. 2018;148:238–52. https://doi.org/10.1016/j.bcp.2018.01.010.
Wang M, Gao M, Yi Z. Biological effects of IL-33/ST2 axis on oral diseases: autoimmune diseases and periodontal diseases. Int Immunopharmacol. 2023;122: 110524. https://doi.org/10.1016/j.intimp.2023.110524.
Milovanovic M, Volarevic V, Radosavljevic G, Jovanovic I, Pejnovic N, Arsenijevic N, Lukic ML. IL-33/ST2 axis in inflammation and immunopathology. Immunol Res. 2012;52(1–2):89–99. https://doi.org/10.1007/s12026-012-8283-9.
Abdul-Wahab GA, Alwan AM, Al-Karawi SI. Evaluation of Serum interleukin-33 level in Iraqi patients with and without periodontal disease. Clin Cosmet Investig Dent. 2024;5(16):201–7. https://doi.org/10.2147/CCIDE.S464951.
Lin M, Gao XL, Li W. IL-33 in patients with periodontitis and chronic obstructive pulmonary disease. Hum Immunol. 2024;15: 110811. https://doi.org/10.1016/j.humimm.2024.110811.
Téllez Corral MA, Daza EH, Jimenez NA, Morales Vera DZ, Velosa Porras J, Latorre Uriza C, Escobar Arregoces FM, Martinez PH, Cortés ME, Otero L, Parra Giraldo CM, Roa Molina NS. Biomarkers for the severity of periodontal disease in patients with obstructive sleep apnea:IL-1 β, IL-6, IL-17A, and IL-33. Heliyon. 2023;9(3): e14340. https://doi.org/10.1016/j.heliyon.2023.e14340.
Renjith A, Rajan NS, Shaila SN. Protein and mRNA expression of interleukin-33 in periodontally diseased and healthy individuals and impact of nonsurgical periodontal therapy in salivary IL-33 levels. J Indian Soc Periodontol. 2023;27(1):45–50. https://doi.org/10.4103/jisp.jisp_390_21.
Yilmaz M, He Q, Demir E, Teräsjärvi J, Gürsoy UK. Salivary IL-33 and sST2 levels in relation to TLR2 rs111200466 polymorphism and periodontitis. Oral Dis. 2024;30(4):2254–61. https://doi.org/10.1111/odi.14675.
Habeeb SAK, Al-Kaabi SJM. Interleukin-33 level in biological fluids for periodontitis patients in AL-Najaf city, Iraq. International Journal of Drug Delivery Technology. 2021;11(3):706–10.
Ballambettu SP, Pradeep AR, Purushottam M, Sen S. Higher interleukin-33 levels in aggressive periodontitis cases. J Indian Soc Periodontol. 2019;23(5):424–9. https://doi.org/10.4103/jisp.jisp_217_19.
Tarrad N, Abdelkawy M, Shaker O. Interleukin-33 and osteoprotegerin levels in gingival crevicular fluid and saliva in chronic periodontitis and their correlation to diabetes mellitus: a cross-sectional study. Perio J. 2018;2(1):1–9.
Sağlam M, Köseoğlu S, Aral CA, Savran L, Pekbağrıyanık T, Çetinkaya A. Increased levels of interleukin-33 in gingival crevicular fluids of patients with chronic periodontitis. Odontology. 2017;105(2):184–90. https://doi.org/10.1007/s10266-016-0259-0.
Kurşunlu SF, Oztürk VÖ, Han B, Atmaca H, Emingil G. Gingival crevicular fluid interleukin-36β (-1F8), interleukin-36γ (-1F9) and interleukin-33 (-1F11) levels in different periodontal disease. Arch Oral Biol. 2015;60(1):77–83. https://doi.org/10.1016/j.archoralbio.2014.08.021.
Papathanasiou E, Teles F, Griffin T, Arguello E, Finkelman M, Hanley J, Theoharides TC. Gingival crevicular fluid levels of interferon-γ, but not interleukin-4 or -33 or thymic stromal lymphopoietin, are increased in inflamed sites in patients with periodontal disease. J Periodontal Res. 2014;49(1):55–61. https://doi.org/10.1111/jre.12078.
Buduneli N, Özçaka Ö, Nalbantsoy A. Interleukin-33 levels in gingival crevicular fluid, saliva, or plasma do not differentiate chronic periodontitis. J Periodontol. 2012;83(3):362–8. https://doi.org/10.1902/jop.2011.110239.
Ladez MAR, Fakour SR, Karbasi M. Evaluation of interleukin 8, 12 & 33 serum level in patients with chronic periodontitis, aggressive periodontitis and healthy subjects. Life Sci J. 2012;9(4):111–7.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;29(372): n71. https://doi.org/10.1136/bmj.n71.
Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, Currie M, Qureshi R, Mattis P, Lisy K, Mu P-F. Chapter 7: Systematic reviews of etiology and risk. In: Aromataris E, Munn Z (Editors). JBI Manual for Evidence Synthesis. JBI, 2020. https://synthesismanual.jbi.global.
Cardoso EM, Reis C, Manzanares-Céspedes MC. Chronic periodontitis, inflammatory cytokines, and interrelationship with other chronic diseases. Postgrad Med. 2018;130(1):98–104. https://doi.org/10.1080/00325481.2018.1396876.
Dosseva-Panova VT, Popova CL, Panov VE. Subgingival microbial profile and production of proinflammatory cytokines in chronic periodontitis. Folia Med. 2014;56(3):152–60. https://doi.org/10.2478/folmed-2014-0022.
Kouzaki H, Iijima K, Kobayashi T, O’Grady SM, Kita H. The danger signal, extracellular ATP, is a sensor for an airborne allergen and triggers IL-33 release and innate Th2-type responses. J Immunol. 2011;186(7):4375–87. https://doi.org/10.4049/jimmunol.1003020.
Kakkar R, Hei H, Dobner S, Lee RT. Interleukin 33 as a mechanically responsive cytokine secreted by living cells. J Biol Chem. 2012;287(9):6941–8. https://doi.org/10.1074/jbc.M111.298703.
Trimarchi M, Lauritano D, Ronconi G, Caraffa A, Gallenga CE, Frydas I, Kritas SK, Calvisi V, Conti P. Mast cell cytokines in acute and chronic gingival tissue inflammation: role of IL-33 and IL-37. Int J Mol Sci. 2022;23(21):13242. https://doi.org/10.3390/ijms232113242.
Tada H, Suzuki R, Nemoto E, Shimauchi H, Matsushita K, Takada H. Increases in IL-33 production by fimbriae and lipopeptide from Porphyromonas gingivalis in mouse bone marrow-derived dendritic cells via Toll-like receptor 2. Biomed Res. 2017;38(3):189–95. https://doi.org/10.2220/biomedres.38.189.
Tada H, Matsuyama T, Nishioka T, Hagiwara M, Kiyoura Y, Shimauchi H, Matsushita K. Porphyromonas gingivalis gingipain-dependently enhances IL-33 production in human gingival epithelial cells. PLoS ONE. 2016;11(4): e0152794. https://doi.org/10.1371/journal.pone.0152794.
Beklen A, Tsaous MG. Interleukin-1 superfamily member, interleukin-33, in periodontal diseases. Biotech Histochem. 2014;89(3):209–14. https://doi.org/10.3109/10520295.2013.832800.
Dong X, Feng J, Li B, Bai D, Xu H. Inhibition of osteoclastogenesis by interleukin-33 administration in the periodontal ligament under mechanical loading. J Periodontal Res. 2022;57(5):1003–13. https://doi.org/10.1111/jre.13039.
Kang H, Yang K, Xiao L, Guo L, Guo C, Yan Y, Qi J, Wang F, Ryffel B, Li C, Deng L. Osteoblast hypoxia-inducible factor-1α pathway activation restrains osteoclastogenesis via the interleukin-33-microrna-34a-notch1 pathway. Front Immunol. 2017;16(8):1312. https://doi.org/10.3389/fimmu.2017.01312.
Lima IL, Macari S, Madeira MF, Rodrigues LF, Colavite PM, Garlet GP, Soriani FM, Teixeira MM, Fukada SY, Silva TA. Osteoprotective effects of IL-33/ST2 link to osteoclast apoptosis. Am J Pathol. 2015;185(12):3338–48. https://doi.org/10.1016/j.ajpath.2015.08.013.
Dandan-Zong Shen C, Liu X, Liu T, Ou Y, Ouyang R. IL-33/ST2 mediating systemic inflammation and neuroinflammation through NF-kB participated in the neurocognitive impairment in obstructive sleep apnea. Int Immunopharmacol. 2023;115:109604. https://doi.org/10.1016/j.intimp.2022.109604.
Tsukasaki M. RANKL and osteoimmunology in periodontitis. J Bone Miner Metab. 2021;39(1):82–90. https://doi.org/10.1007/s00774-020-01165-3.
Malcolm J, Awang RA, Oliver-Bell J, Butcher JP, Campbell L, Adrados Planell A, Lappin DF, Fukada SY, Nile CJ, Liew FY, Culshaw S. IL-33 exacerbates periodontal disease through induction of RANKL. J Dent Res. 2015;94(7):968–75. https://doi.org/10.1177/0022034515577815.
Torrungruang K, Katudat D, Mahanonda R, Sritara P, Udomsak A. Periodontitis is associated with elevated serum levels of cardiac biomarkers-soluble ST2 and C-reactive protein. J Clin Periodontol. 2019;46(8):809–18. https://doi.org/10.1111/jcpe.13149.
Ozgur E, Topcu DI, Bayraktar N, Alptekin NO. Peri-implant crevicular fluid and serum levels of soluble ST2 in peri-implant diseases: a pilot study. J Periodontal Res. 2023;58(1):204–11. https://doi.org/10.1111/jre.13082.
Navya PD, Kaarthikeyan G, Raj JS, Alamoudi A, Bahammam MA, Zidane B, Bahammam HA, Bahammam SA, Hassan AAA, Kamil MA, Bhandi S, Raj AT, Patil S. Suppression of tumorigenicity 2 pro-inflammatory biomarker linking diabetes mellitus and periodontitis: a pilot study. Med Sci Monit. 2022;15(28): e938218. https://doi.org/10.12659/MSM.938218.
Acknowledgements
None.
Funding
No external funding was received.
Author information
Authors and Affiliations
Contributions
Conceptualization, M.A.A.-S.; methodology, M.A.A.-S-.; software, M.A.A.-S.; validation, M.A.A.-S, N.S.R.-C, S.R.-F, E.U.S.-T and A.H.; formal analysis, M.A.A.-S.; investigation, M.A.A.-S.; resources, M.A.A.-S.; data curation, M.A.A.-S.; writing—original draft preparation, M.A.A.-S.; writing—review and editing, M.A.A.-S, N.S.R.-C, S.R.-F, E.U.S.-T and A.H.; visualization, M.A.A.-S, N.S.R.-C, S.R.-F, E.U.S.-T and A.H.; supervision, M.A.A.-S, N.S.R.-C, S.R.-F, E.U.S.-T and A.H.; project administration, M.A.A.-S. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Alarcón-Sánchez, M.A., Romero-Castro, N.S., Reyes-Fernández, S. et al. Expression of IL-33 in subjects with periodontitis: a systematic review and meta-analysis. Eur J Med Res 29, 440 (2024). https://doi.org/10.1186/s40001-024-02039-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40001-024-02039-4